Mise en page 1 - Laboratoire National des Champs Magnétiques ...
Mise en page 1 - Laboratoire National des Champs Magnétiques ...
Mise en page 1 - Laboratoire National des Champs Magnétiques ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
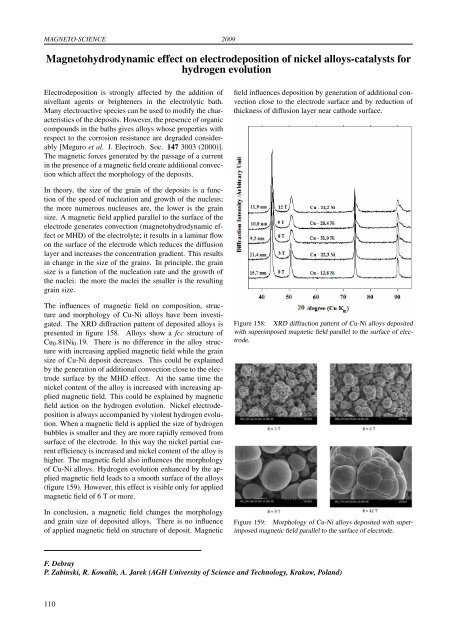
MAGNETO-SCIENCE 2009Magnetohydrodynamic effect on electrodeposition of nickel alloys-catalysts forhydrog<strong>en</strong> evolutionElectrodeposition is strongly affected by the addition ofnivellant ag<strong>en</strong>ts or bright<strong>en</strong>ers in the electrolytic bath.Many electroactive species can be used to modify the characteristicsof the deposits. However, the pres<strong>en</strong>ce of organiccompounds in the baths gives alloys whose properties withrespect to the corrosion resistance are degraded considerably[Meguro et al. J. Electroch. Soc. 147 3003 (2000)].The magnetic forces g<strong>en</strong>erated by the passage of a curr<strong>en</strong>tin the pres<strong>en</strong>ce of a magnetic field create additional convectionwhich affect the morphology of the deposits.field influ<strong>en</strong>ces deposition by g<strong>en</strong>eration of additional convectionclose to the electrode surface and by reduction ofthickness of diffusion layer near cathode surface.In theory, the size of the grain of the deposits is a functionof the speed of nucleation and growth of the nucleus;the more numerous nucleuses are, the lower is the grainsize. A magnetic field applied parallel to the surface of theelectrode g<strong>en</strong>erates convection (magnetohydrodynamic effector MHD) of the electrolyte; it results in a laminar flowon the surface of the electrode which reduces the diffusionlayer and increases the conc<strong>en</strong>tration gradi<strong>en</strong>t. This resultsin change in the size of the grains. In principle, the grainsize is a function of the nucleation rate and the growth ofthe nuclei: the more the nuclei the smaller is the resultinggrain size.The influ<strong>en</strong>ces of magnetic field on composition, structureand morphology of Cu-Ni alloys have be<strong>en</strong> investigated.The XRD diffraction pattern of deposited alloys ispres<strong>en</strong>ted in figure 158. Alloys show a fcc structure ofCu 0 .81Ni 0 .19. There is no differ<strong>en</strong>ce in the alloy structurewith increasing applied magnetic field while the grainsize of Cu-Ni deposit decreases. This could be explainedby the g<strong>en</strong>eration of additional convection close to the electro<strong>des</strong>urface by the MHD effect. At the same time th<strong>en</strong>ickel cont<strong>en</strong>t of the alloy is increased with increasing appliedmagnetic field. This could be explained by magneticfield action on the hydrog<strong>en</strong> evolution. Nickel electrodepositionis always accompanied by viol<strong>en</strong>t hydrog<strong>en</strong> evolution.Wh<strong>en</strong> a magnetic field is applied the size of hydrog<strong>en</strong>bubbles is smaller and they are more rapidly removed fromsurface of the electrode. In this way the nickel partial curr<strong>en</strong>teffici<strong>en</strong>cy is increased and nickel cont<strong>en</strong>t of the alloy ishigher. The magnetic field also influ<strong>en</strong>ces the morphologyof Cu-Ni alloys. Hydrog<strong>en</strong> evolution <strong>en</strong>hanced by the appliedmagnetic field leads to a smooth surface of the alloys(figure 159). However, this effect is visible only for appliedmagnetic field of 6 T or more.In conclusion, a magnetic field changes the morphologyand grain size of deposited alloys. There is no influ<strong>en</strong>ceof applied magnetic field on structure of deposit. MagneticFigure 158: XRD diffraction pattern of Cu-Ni alloys depositedwith superimposed magnetic field parallel to the surface of electrode.Figure 159: Morphology of Cu-Ni alloys deposited with superimposedmagnetic field parallel to the surface of electrode.F. DebrayP. Zabinski, R. Kowalik, A. Jarek (AGH University of Sci<strong>en</strong>ce and Technology, Krakow, Poland)110