2009 METALS, SUPERCONDUCTORS...Coexist<strong>en</strong>ce of closed orbit and quantum interferometer with the same crosssection in the organic metal β”-(BEDT-TTF) 4 (H 3 O)[Fe(C 2 O 4 ) 3 ]·C 6 H 4 Cl 2The family of quasi-two-dim<strong>en</strong>sional charge transfer saltsβ”-(BEDT-TTF) 4 (A)[M(C 2 O 4 ) 3 ]Solv (where BEDT-TTFstands for bis-ethyl<strong>en</strong>edithio-tetrathiafulval<strong>en</strong>e, A is amonoval<strong>en</strong>t cation, M is a trival<strong>en</strong>t cation and Solv is asolv<strong>en</strong>t) have raised great interest in particular because ityielded, more than t<strong>en</strong> years ago, the first organic superconductorat ambi<strong>en</strong>t pressure with magnetic ions.due to the relatively narrow field range in which these latteroscillations can be observed (B > 20 T).The Lifshitzs-Kosevich formalism accounts for the fieldand temperature dep<strong>en</strong>d<strong>en</strong>ce of both the SdH and dHvAdata over all the explored range. However, a very weak thermaldamping of the Fourier compon<strong>en</strong>t F b , with the highestamplitude, is evid<strong>en</strong>ced for SdH spectra above about 6 K(see figure 95). As a result, magnetoresistance oscillationsare observed at temperatures higher than 30 K. Taking intoaccount the temperature dep<strong>en</strong>d<strong>en</strong>ce of the scattering rate,this feature, which is not observed for dHvA oscillations(recorded up to 15 K), is in line with the coexist<strong>en</strong>ce, at leastin the temperature range around 6 K, of a closed orbit b anda symmetric (i.e. with a zero effective mass) quantum interfer<strong>en</strong>cepath with the same area (keeping in mind that dHvAoscillations are only s<strong>en</strong>sitive to the d<strong>en</strong>sity of states). Thisresult, which cannot be interpreted in the framework of theFermi surface displayed in figure 94(d), points to a Fermisurface reconstruction in this compound. For details, see[Vignolles et al. Eur. Phys. J. B 71 203 (2009)].Figure 94: (a) Field-dep<strong>en</strong>d<strong>en</strong>t interlayer resistance ofβ”-(BEDT-TTF) 4 (H 3 O)[Fe(C 2 O 4 ) 3 ]·C 6 H 4 Cl 2 for θ = 0 ◦ (θ is theangle betwe<strong>en</strong> the field direction and the normal to the conductingplane). (b) Fourier analysis deduced from the oscillatory part ofthe magnetoresistance displayed in the inset. The field range is18-54 T. Marks are calculated with F a = 74 T and F b = 348 T. (c)Magnetic torque at θ = 29 ◦ . Corresponding Fourier analysis aredisplayed in the inset. The field range is 30-53.5 T and 38-53.5 Tbelow and above 9 K, respectively. (d) Textbook case of Fermisurface accounting for the frequ<strong>en</strong>cies a, b and b-a.Magnetoresistance and magnetic torque of the salt with A= H 3 O + , M = Fe 3+ and Solv = C 6 H 4 Cl 2 have be<strong>en</strong> investigatedin pulsed magnetic fields of up to 54 T. Shubnikov-deHaas (SdH) oscillations reveal three basic frequ<strong>en</strong>cies F a ,F b and F b−a , which, in line with band structure calculations,can be interpreted on the basis of three comp<strong>en</strong>sated closedorbits originating from a hole orbit with an area equal to thatof the first Brillouin zone (see figure 94). Only F a and F bare observed in de Haas-van Alph<strong>en</strong> (dHvA) spectra, likelyFigure 95: Temperature dep<strong>en</strong>d<strong>en</strong>ce of the amplitude of the boscillations for dHvA and SdH data. Empty and solid symbolscorrespond to a mean field value of 44.6 T and 30 T/cos(θ), respectively(θ is the angle betwe<strong>en</strong> the field direction and the normal tothe conducting plane). Solid lines are best fits of the Lifshitzs-Kosevichformula. A zero-effective mass and a temperature-dep<strong>en</strong>d<strong>en</strong>tscattering rate are considered for the SdH data in the hightemperature range.D. Vignolles, A. AudouardV.N. Laukhin, E. Canadell (ICMAB, Barcelona, Spain), E.B. Yagubskii (IPCP, Chernogolovka, Russian Federation)69
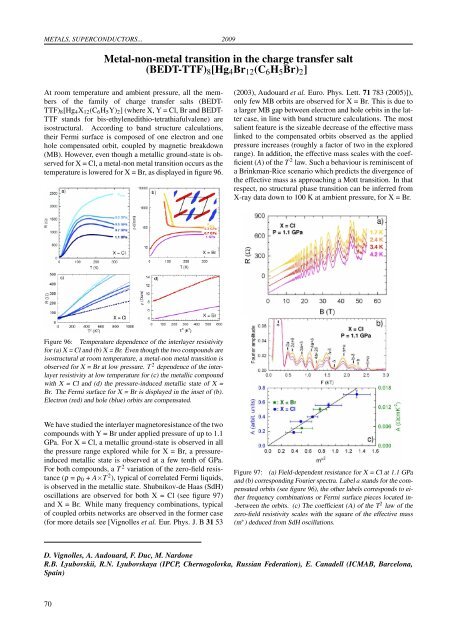
METALS, SUPERCONDUCTORS... 2009Metal-non-metal transition in the charge transfer salt(BEDT-TTF) 8 [Hg 4 Br 12 (C 6 H 5 Br) 2 ]At room temperature and ambi<strong>en</strong>t pressure, all the membersof the family of charge transfer salts (BEDT-TTF) 8 [Hg 4 X 12 (C 6 H 5 Y) 2 ] (where X, Y = Cl, Br and BEDT-TTF stands for bis-ethyl<strong>en</strong>edithio-tetrathiafulval<strong>en</strong>e) areisostructural. According to band structure calculations,their Fermi surface is composed of one electron and onehole comp<strong>en</strong>sated orbit, coupled by magnetic breakdown(MB). However, ev<strong>en</strong> though a metallic ground-state is observedfor X = Cl, a metal-non metal transition occurs as thetemperature is lowered for X = Br, as displayed in figure 96.(2003), Audouard et al. Euro. Phys. Lett. 71 783 (2005)]),only few MB orbits are observed for X = Br. This is due toa larger MB gap betwe<strong>en</strong> electron and hole orbits in the lattercase, in line with band structure calculations. The mostsali<strong>en</strong>t feature is the sizeable decrease of the effective masslinked to the comp<strong>en</strong>sated orbits observed as the appliedpressure increases (roughly a factor of two in the exploredrange). In addition, the effective mass scales with the coeffici<strong>en</strong>t(A) of the T 2 law. Such a behaviour is reminisc<strong>en</strong>t ofa Brinkman-Rice sc<strong>en</strong>ario which predicts the diverg<strong>en</strong>ce ofthe effective mass as approaching a Mott transition. In thatrespect, no structural phase transition can be inferred fromX-ray data down to 100 K at ambi<strong>en</strong>t pressure, for X = Br.Figure 96: Temperature dep<strong>en</strong>d<strong>en</strong>ce of the interlayer resistivityfor (a) X = Cl and (b) X = Br. Ev<strong>en</strong> though the two compounds areisostructural at room temperature, a metal-non metal transition isobserved for X = Br at low pressure. T 2 dep<strong>en</strong>d<strong>en</strong>ce of the interlayerresistivity at low temperature for (c) the metallic compoundwith X = Cl and (d) the pressure-induced metallic state of X =Br. The Fermi surface for X = Br is displayed in the inset of (b).Electron (red) and hole (blue) orbits are comp<strong>en</strong>sated.We have studied the interlayer magnetoresistance of the twocompounds with Y = Br under applied pressure of up to 1.1GPa. For X = Cl, a metallic ground-state is observed in allthe pressure range explored while for X = Br, a pressureinducedmetallic state is observed at a few t<strong>en</strong>th of GPa.For both compounds, a T 2 variation of the zero-field resistance(ρ = ρ 0 + A×T 2 ), typical of correlated Fermi liquids,is observed in the metallic state. Shubnikov-de Haas (SdH)oscillations are observed for both X = Cl (see figure 97)and X = Br. While many frequ<strong>en</strong>cy combinations, typicalof coupled orbits networks are observed in the former case(for more details see [Vignolles et al. Eur. Phys. J. B 31 53Figure 97: (a) Field-dep<strong>en</strong>d<strong>en</strong>t resistance for X = Cl at 1.1 GPaand (b) corresponding Fourier spectra. Label a stands for the comp<strong>en</strong>satedorbits (see figure 96), the other labels corresponds to eitherfrequ<strong>en</strong>cy combinations or Fermi surface pieces located in--betwe<strong>en</strong> the orbits. (c) The coeffici<strong>en</strong>t (A) of the T 2 law of thezero-field resistivity scales with the square of the effective mass(m ∗ ) deduced from SdH oscillations.D. Vignolles, A. Audouard, F. Duc, M. NardoneR.B. Lyubovskii, R.N. Lyubovskaya (IPCP, Chernogolovka, Russian Federation), E. Canadell (ICMAB, Barcelona,Spain)70
- Page 1 and 2:
LABORATOIRE NATIONAL DES CHAMPS MAG
- Page 4 and 5:
TABLE OF CONTENTSPreface 1Carbon Al
- Page 6 and 7:
Coexistence of closed orbit and qua
- Page 8:
2009PrefaceDear Reader,You have bef
- Page 12 and 13:
2009 CARBON ALLOTROPESInvestigation
- Page 14 and 15:
2009 CARBON ALLOTROPESPropagative L
- Page 16 and 17:
2009 CARBON ALLOTROPESEdge fingerpr
- Page 18 and 19:
2009 CARBON ALLOTROPESObservation o
- Page 20 and 21:
2009 CARBON ALLOTROPESImproving gra
- Page 22 and 23:
2009 CARBON ALLOTROPESHow perfect c
- Page 24 and 25:
2009 CARBON ALLOTROPESTuning the el
- Page 26 and 27: 2009 CARBON ALLOTROPESElectric fiel
- Page 28 and 29: 2009 CARBON ALLOTROPESMagnetotransp
- Page 30 and 31: 2009 CARBON ALLOTROPESGraphite from
- Page 32: 2009Two-Dimensional Electron Gas25
- Page 35 and 36: TWO-DIMENSIONAL ELECTRON GAS 2009Di
- Page 37 and 38: TWO-DIMENSIONAL ELECTRON GAS 2009Sp
- Page 39 and 40: TWO-DIMENSIONAL ELECTRON GAS 2009Cr
- Page 41 and 42: TWO-DIMENSIONAL ELECTRON GAS 2009Re
- Page 43 and 44: TWO-DIMENSIONAL ELECTRON GAS 2009In
- Page 45 and 46: TWO-DIMENSIONAL ELECTRON GAS 2009Ho
- Page 47 and 48: TWO-DIMENSIONAL ELECTRON GAS 2009Te
- Page 50 and 51: 2009 SEMICONDUCTORS AND NANOSTRUCTU
- Page 52 and 53: 2009 SEMICONDUCTORS AND NANOSTRUCTU
- Page 54 and 55: 2009 SEMICONDUCTORS AND NANOSTRUCTU
- Page 56 and 57: 2009 SEMICONDUCTORS AND NANOSTRUCTU
- Page 58 and 59: 2009 SEMICONDUCTORS AND NANOSTRUCTU
- Page 60: 2009Metals, Superconductors and Str
- Page 63 and 64: METALS, SUPERCONDUCTORS... 2009Anom
- Page 65 and 66: METALS, SUPERCONDUCTORS... 2009Magn
- Page 67 and 68: METALS, SUPERCONDUCTORS ... 2009Coe
- Page 69 and 70: METALS, SUPERCONDUCTORS ... 2009Fie
- Page 71 and 72: METALS, SUPERCONDUCTORS... 2009High
- Page 73 and 74: METALS, SUPERCONDUCTORS... 2009Angu
- Page 75: METALS, SUPERCONDUCTORS... 2009Magn
- Page 79 and 80: METALS, SUPERCONDUCTORS... 2009Temp
- Page 81 and 82: METALS, SUPERCONDUCTORS... 200974
- Page 84 and 85: 2009 MAGNETIC SYSTEMSY b 3+ → Er
- Page 86 and 87: 2009 MAGNETIC SYSTEMSMagnetotranspo
- Page 88 and 89: 2009 MAGNETIC SYSTEMSHigh field tor
- Page 90 and 91: 2009 MAGNETIC SYSTEMSNuclear magnet
- Page 92 and 93: 2009 MAGNETIC SYSTEMSStructural ana
- Page 94 and 95: 2009 MAGNETIC SYSTEMSEnhancement ma
- Page 96 and 97: 2009 MAGNETIC SYSTEMSInvestigation
- Page 98 and 99: 2009 MAGNETIC SYSTEMSField-induced
- Page 100 and 101: 2009 MAGNETIC SYSTEMSMagnetic prope
- Page 102: 2009Biology, Chemistry and Soft Mat
- Page 105 and 106: BIOLOGY, CHEMISTRY AND SOFT MATTER
- Page 108 and 109: 2009 APPLIED SUPERCONDUCTIVITYMagne
- Page 110 and 111: 2009 APPLIED SUPERCONDUCTIVITYPhtha
- Page 112: 2009Magneto-Science105
- Page 115 and 116: MAGNETO-SCIENCE 2009Study of the in
- Page 117 and 118: MAGNETO-SCIENCE 2009Magnetohydrodyn
- Page 119 and 120: MAGNETO-SCIENCE 2009112
- Page 122 and 123: 2009 MAGNET DEVELOPMENT AND INSTRUM
- Page 124 and 125: 2009 MAGNET DEVELOPMENT AND INSTRUM
- Page 126 and 127:
2009 MAGNET DEVELOPMENT AND INSTRUM
- Page 128 and 129:
2009 MAGNET DEVELOPMENT AND INSTRUM
- Page 130 and 131:
2009 MAGNET DEVELOPMENT AND INSTRUM
- Page 132 and 133:
2009 MAGNET DEVELOPMENT AND INSTRUM
- Page 134 and 135:
2009 MAGNET DEVELOPMENT AND INSTRUM
- Page 136 and 137:
2009 PROPOSALSProposals for Magnet
- Page 138 and 139:
2009 PROPOSALSSpin-Jahn-Teller effe
- Page 140 and 141:
2009 PROPOSALSQuantum Oscillations
- Page 142 and 143:
2009 PROPOSALSThermoelectric tensor
- Page 144 and 145:
2009 PROPOSALSDr. EscoffierCyclotro
- Page 146 and 147:
2009 PROPOSALSHigh field magnetotra
- Page 148 and 149:
2009 THESESPhD Theses 20091. Nanot
- Page 150 and 151:
2009 PUBLICATIONS[21] O. Drachenko,
- Page 152 and 153:
2009 PUBLICATIONS[75] S. Nowak, T.
- Page 154 and 155:
Contributors of the LNCMI to the Pr
- Page 156 and 157:
Institut Jean Lamour, Nancy : 68Ins
- Page 158 and 159:
Lawrence Berkeley National Laborato