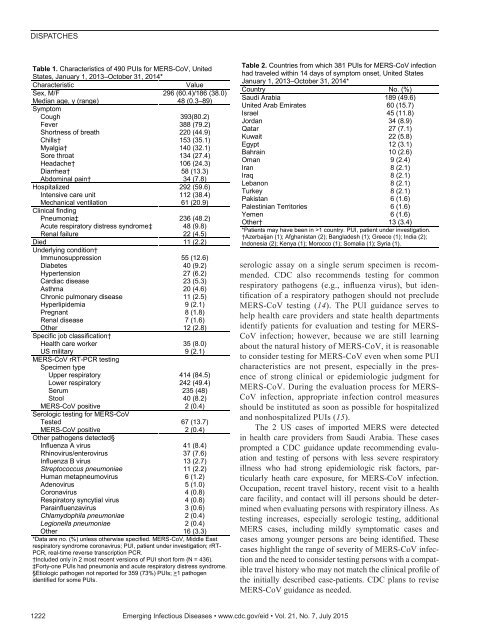
DISPATCHESTable 1. Characteristics of 490 PUIs for MERS-CoV, UnitedStates, January 1, 2013–October 31, 2014*CharacteristicValueSex, M/F 296 (60.4)/186 (38.0)Median age, y (range) 48 (0.3–89)SymptomCough 393(80.2)Fever 388 (79.2)Shortness of breath 220 (44.9)Chills† 153 (35.1)Myalgia† 140 (32.1)Sore throat 134 (27.4)Headache† 106 (24.3)Diarrhea† 58 (13.3)Abdominal pain† 34 (7.8)Hospitalized 292 (59.6)Intensive care unit 112 (38.4)Mechanical ventilation 61 (20.9)Clinical findingPneumonia‡ 236 (48.2)Acute respiratory distress syndrome‡ 48 (9.8)Renal failure 22 (4.5)Died 11 (2.2)Underlying condition†Immunosuppression 55 (12.6)Diabetes 40 (9.2)Hypertension 27 (6.2)Cardiac disease 23 (5.3)Asthma 20 (4.6)Chronic pulmonary disease 11 (2.5)Hyperlipidemia 9 (2.1)Pregnant 8 (1.8)Renal disease 7 (1.6)Other 12 (2.8)Specific job classification†Health care worker 35 (8.0)US military 9 (2.1)MERS-CoV rRT-PCR testingSpecimen typeUpper respiratory 414 (84.5)Lower respiratory 242 (49.4)Serum 235 (48)Stool 40 (8.2)MERS-CoV positive 2 (0.4)Serologic testing for MERS-CoVTested 67 (13.7)MERS-CoV positive 2 (0.4)Other pathogens detected§Influenza A virus 41 (8.4)Rhinovirus/enterovirus 37 (7.6)Influenza B virus 13 (2.7)Streptococcus pneumoniae 11 (2.2)Human metapneumovirus 6 (1.2)Adenovirus 5 (1.0)Coronavirus 4 (0.8)Respiratory syncytial virus 4 (0.8)Parainfluenzavirus 3 (0.6)Chlamydophila pneumoniae 2 (0.4)Legionella pneumoniae 2 (0.4)Other 16 (3.3)*Data are no. (%) unless otherwise specified. MERS-CoV, Middle Eastrespiratory syndrome coronavirus; PUI, patient under investigation; rRT-PCR, real-time reverse transcription PCR.†Included only in 2 most recent <strong>version</strong>s of PUI short form (N = 436).‡Forty-one PUIs had pneumonia and acute respiratory distress syndrome.§Etiologic pathogen not reported for 359 (73%) PUIs; >1 pathogenidentified for some PUIs.Table 2. Countries from which 381 PUIs for MERS-CoV infectionhad traveled within 14 days of symptom onset, United StatesJanuary 1, 2013–October 31, 2014*Country No. (%)Saudi Arabia 189 (49.6)United Arab Emirates 60 (15.7)Israel 45 (11.8)Jordan 34 (8.9)Qatar 27 (7.1)Kuwait 22 (5.8)Egypt 12 (3.1)Bahrain 10 (2.6)Oman 9 (2.4)Iran 8 (2.1)Iraq 8 (2.1)Lebanon 8 (2.1)Turkey 8 (2.1)Pakistan 6 (1.6)Palestinian Territories 6 (1.6)Yemen 6 (1.6)Other† 13 (3.4)*Patients may have been in >1 country. PUI, patient under investigation.†Azerbaijan (1); Afghanistan (2); Bangladesh (1); Greece (1); India (2);Indonesia (2); Kenya (1); Morocco (1); Somalia (1); Syria (1).serologic assay on a single serum specimen is recommended.CDC also recommends testing for commonrespiratory pathogens (e.g., influenza virus), but identificationof a respiratory pathogen should not precludeMERS-CoV testing (14). The PUI guidance serves tohelp health care providers and state health departmentsidentify patients for evaluation and testing for MERS-CoV infection; however, because we are still learningabout the natural history of MERS-CoV, it is reasonableto consider testing for MERS-CoV even when some PUIcharacteristics are not present, especially in the presenceof strong clinical or epidemiologic judgment forMERS-CoV. During the evaluation process for MERS-CoV infection, appropriate infection control measuresshould be instituted as soon as possible for hospitalizedand nonhospitalized PUIs (15).The 2 US cases of imported MERS were detectedin health care providers from Saudi Arabia. These casesprompted a CDC guidance update recommending evaluationand testing of persons with less severe respiratoryillness who had strong epidemiologic risk factors, particularlyheath care exposure, for MERS-CoV infection.Occupation, recent travel history, recent visit to a healthcare facility, and contact will ill persons should be determinedwhen evaluating persons with respiratory illness. Astesting increases, especially serologic testing, additionalMERS cases, including mildly symptomatic cases andcases among younger persons are being identified. Thesecases highlight the range of severity of MERS-CoV infectionand the need to consider testing persons with a compatibletravel history who may not match the clinical profile ofthe initially described case-patients. CDC plans to reviseMERS-CoV guidance as needed.1222 Emerging Infectious Diseases • www.cdc.gov/eid • Vol. 21, No. 7, July 2015
Patients under investigation for MERS-CoVAcknowledgmentWe thank the state and local health departments, healthcareproviders, CDC Emergency Operations Center, and CDC MERSDomestic Response Team for all of their hard work on MERS-CoV, especially in evaluating, testing, and reporting PUIs.Dr. Schneider is a senior medical epidemiologist with theDivision of Viral Diseases, CDC. Her current research interestsinclude respiratory viruses.References1. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME,Fouchier RAM. Isolation of a novel coronavirus from a man withpneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–20.http://dx.doi.org/10.1056/NEJMoa12117212. Milne-Price S, Miazgowicz KL, Munster VJ. The emergence ofthe Middle East respiratory syndrome coronavirus. Pathog Dis.2014;71:121–36. http://dx.doi.org/10.1111/2049-632X.121663. Raj VS, Osterhaus A. DME, Fouchier R AM, Haagmans BL.MERS: emergence of a novel human coronavirus. Curr Opin Virol.2014;5:58–62. http://dx.doi.org/10.1016/j.coviro.2014.01.0104. World Health Organization. Middle East respiratory syndromecoronavirus (MERS-CoV): summary of current situation, literatureupdate and risk assessment—as of 5 February 2015 [cited 2015Mar 10]. http://www.who.int/csr/disease/coronavirus_infections/mers-5-february-2015.<strong>pdf</strong>?ua=15. Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA,Al-Hajjar S, Al-Barrak A, et al. Epidemiological, demographic,and clinical characteristics of 47 cases of Middle East respiratorysyndrome coronavirus disease from Saudi Arabia: a descriptivestudy. Lancet Infect Dis. 2013;13:752–61. http://dx.doi.org/10.1016/S1473-3099(13)70204-46. Drosten C, Seilmaier M, Corman VM, Hartmann W, Scheible G,Sack S, et al. Clinical features and virological analysis of a case ofMiddle East respiratory syndrome coronavirus infection. LancetInfect Dis. 2013;13:745–51. http://dx.doi.org/10.1016/S1473-3099(13)70154-37. Omrani AS, Matin MA, Haddad Q, Al-Nakhli D, Memish ZA,Albarrak AM. A family cluster of Middle East respiratorysyndrome coronavirus infections related to a likely unrecognizedasymptomatic or mild case. Int J Infect Dis. 2013;17:e668–72.http://dx.doi.org/10.1016/j.ijid.2013.07.0018. Memish ZA, Mishra N, Olival KJ, Fagbo SF, Kapoor V,Epstein JH, et al. Middle East respiratory syndrome coronavirus inbats, Saudi Arabia. Emerg Infect Dis. 2013;19:1819–23.http://dx.doi.org/10.3201/eid1911.1311729. Reusken CB, Farag EA, Jonges M, Godeke GJ, El-Sayed AM,Pas SD, et al. Middle East respiratory syndrome coronavirus(MERS-CoV) RNA and neutralising antibodies in milk collectedaccording to local customs from dromedary camels, Qatar, April2014. Euro Surveill. 2014;19:20829.10. Lu X, Whitaker B, Sakthivel SKK, Kamili S, Rose LE, Lowe L,et al. Real-time transcription-PCR assay panel for Middle Eastrespiratory syndrome coronavirus. J Clin Microbiol. 2014;52:67–75. http://dx.doi.org/10.1128/JCM.02533-1311. Centers for Disease Control and Prevention. Middle Eastrespiratory syndrome (MERS). Data collection [cited 2015Mar 10]. http://www.cdc.gov/coronavirus/mers/data-collection.html12. Bialek SR, Allen D, Alvarado-Ramy F, Arthur R, Balajee A,Bell D, et al. First confirmed cases of Middle East respiratorysyndrome coronavirus (MERS-CoV) infection in the United States,updated information on the epidemiology of MERS-CoV infection,and guidance for the public, clinicians, and public healthauthorities—May 2014. MMWR Morb Mortal Wkly Rep. 2014;63:431–6.13. Kapoor M, Pringle K, Kumar A, Dearth S, Liu L, Lovchik J, et al.Clinical and laboratory findings of the first imported case of MiddleEast respiratory syndrome coronavirus (MERS-CoV) in the UnitedStates. Clin Infect Dis. 2014;59:1511–8. http://dx.doi.org/10.1093/cid/ciu63514. Centers for Disease Control and Prevention. Interim guidelinesfor collecting, handling, and testing clinical specimens frompatients under investigation (PUIs) for Middle East respiratorysyndrome coronavirus (MERS-CoV)—<strong>version</strong> 2[cited 2015 Mar 10] http://www.cdc.gov/coronavirus/mers/guidelines-clinical-specimens.html15. Centers for Disease Control and Prevention. Interim infectionprevention and control recommendations for hospitalized patientswith Middle East respiratory syndrome coronavirus (MERS-CoV)[cited 2015 Mar 10]. http://www.cdc.gov/coronavirus/mers/infection-prevention-control.htmlAddress for correspondence: Eileen Schneider, Centers for DiseaseControl and Prevention, 1600 Clifton Rd NE, Mailstop A34, Atlanta,GA, 30329-4027, USA; email: ees2@cdc.govEID Podcast: Carbapenem-Resistant EnterobacteriaceaeDr. Mike Miller reads anabridged <strong>version</strong> of the article,Deaths Attributable toCarbapenem-ResistantEnterobacteriaceaeInfectionshttp://www2c.cdc.gov/podcasts/player.asp?f=8633574Emerging Infectious Diseases • www.cdc.gov/eid • Vol. 21, No. 7, July 2015 1223
- Page 3 and 4:
July 2015SynopsisOn the CoverMarian
- Page 5 and 6:
1240 Gastroenteritis OutbreaksCause
- Page 7 and 8:
SYNOPSISDisseminated Infections wit
- Page 9 and 10:
Disseminated Infections with Talaro
- Page 11 and 12:
Disseminated Infections with Talaro
- Page 13 and 14:
Macacine Herpesvirus 1 inLong-Taile
- Page 15 and 16:
Macacine Herpesvirus 1 in Macaques,
- Page 17 and 18:
Macacine Herpesvirus 1 in Macaques,
- Page 19:
Macacine Herpesvirus 1 in Macaques,
- Page 23:
Malaria among Young Infants, Africa
- Page 26 and 27:
RESEARCHFigure 3. Dynamics of 19-kD
- Page 28 and 29:
Transdermal Diagnosis of MalariaUsi
- Page 30 and 31:
RESEARCHFigure 2. A) Acoustic trace
- Page 32 and 33:
RESEARCHof malaria-infected mosquit
- Page 34 and 35:
Lack of Transmission amongClose Con
- Page 36 and 37:
RESEARCH(IFA) and microneutralizati
- Page 38 and 39:
RESEARCHoropharyngeal, and serum sa
- Page 40 and 41:
RESEARCH6. Assiri A, McGeer A, Perl
- Page 42 and 43:
RESEARCHadvanced genomic sequencing
- Page 44 and 45:
RESEARCHTable 2. Next-generation se
- Page 46 and 47:
RESEARCHTable 3. Mutation analysis
- Page 48 and 49:
RESEARCHReferences1. Baize S, Panne
- Page 50 and 51:
Parechovirus Genotype 3 Outbreakamo
- Page 52 and 53:
RESEARCHFigure 1. Venn diagramshowi
- Page 54 and 55:
RESEARCHTable 2. HPeV testing of sp
- Page 56 and 57:
RESEARCHFigure 5. Distribution of h
- Page 58 and 59:
RESEARCHReferences1. Selvarangan R,
- Page 60 and 61:
RESEARCHthe left lobe was sampled b
- Page 62 and 63:
RESEARCHTable 2. Middle East respir
- Page 64 and 65:
RESEARCHseroprevalence in domestic
- Page 66 and 67:
RESEARCHmeasure their current surve
- Page 68 and 69:
RESEARCHTable 2. States with labora
- Page 70 and 71:
RESEARCHFigure 2. Comparison of sur
- Page 72 and 73:
RESEARCH9. Centers for Disease Cont
- Page 74 and 75:
RESEARCHthe analyses. Cases in pers
- Page 76 and 77:
RESEARCHTable 3. Sampling results (
- Page 78 and 79: RESEARCHpresence of Legionella spp.
- Page 80 and 81: Seroprevalence for Hepatitis Eand O
- Page 82 and 83: RESEARCHTable 1. Description of stu
- Page 84 and 85: RESEARCHTable 3. Crude and adjusted
- Page 86 and 87: RESEARCHrates by gender or HIV stat
- Page 88 and 89: RESEARCH25. Taha TE, Kumwenda N, Ka
- Page 90 and 91: POLICY REVIEWDutch Consensus Guidel
- Page 92 and 93: POLICY REVIEWTable 3. Comparison of
- Page 94 and 95: POLICY REVIEW6. Botelho-Nevers E, F
- Page 96 and 97: DISPATCHESFigure 1. Phylogenetic tr
- Page 98 and 99: DISPATCHESSevere Pediatric Adenovir
- Page 100 and 101: DISPATCHESTable 1. Demographics and
- Page 102 and 103: DISPATCHES13. Kim YJ, Hong JY, Lee
- Page 104 and 105: DISPATCHESTable. Alignment of resid
- Page 106 and 107: DISPATCHESFigure 2. Interaction of
- Page 108 and 109: DISPATCHESSchmallenberg Virus Recur
- Page 110 and 111: DISPATCHESFigure 2. Detection of Sc
- Page 112 and 113: DISPATCHESFigure 1. Histopathologic
- Page 114: DISPATCHESFigure 2. Detection of fo
- Page 117 and 118: Influenza Virus Strains in the Amer
- Page 119 and 120: Novel Arenavirus Isolates from Nama
- Page 121 and 122: Novel Arenaviruses, Southern Africa
- Page 123 and 124: Readability of Ebola Informationon
- Page 125 and 126: Readability of Ebola Information on
- Page 127: Patients under investigation for ME
- Page 131 and 132: Wildlife Reservoir for Hepatitis E
- Page 133 and 134: Asymptomatic Malaria and Other Infe
- Page 135 and 136: Asymptomatic Malaria in Children fr
- Page 137 and 138: Bufavirus in Wild Shrews and Nonhum
- Page 139 and 140: Bufavirus in Wild Shrews and Nonhum
- Page 141 and 142: Range Expansion for Rat Lungworm in
- Page 143 and 144: Slow Clearance of Plasmodium falcip
- Page 145 and 146: Slow Clearance of Plasmodium falcip
- Page 147 and 148: Gastroenteritis Caused by Norovirus
- Page 149 and 150: Ebola Virus Stability on Surfaces a
- Page 151 and 152: Ebola Virus Stability on Surfaces a
- Page 153 and 154: Outbreak of Ciprofloxacin-Resistant
- Page 155 and 156: Outbreak of S. sonnei, South KoreaT
- Page 157 and 158: Rapidly Expanding Range of Highly P
- Page 159 and 160: Cluster of Ebola Virus Disease, Bon
- Page 161 and 162: Cluster of Ebola Virus Disease, Lib
- Page 163 and 164: ANOTHER DIMENSIONThe Past Is Never
- Page 165 and 166: Measles Epidemic, Boston, Massachus
- Page 167 and 168: LETTERSInfluenza A(H5N6)Virus Reass
- Page 169 and 170: LETTERSsystem (8 kb-span paired-end
- Page 171 and 172: LETTERS3. Van Hong N, Amambua-Ngwa
- Page 173 and 174: LETTERSTable. Prevalence of Bartone
- Page 175 and 176: LETTERSavian influenza A(H5N1) viru
- Page 177 and 178: LETTERSprovinces and a total of 200
- Page 179 and 180:
LETTERS7. Manian FA. Bloodstream in
- Page 181 and 182:
LETTERSforward projections. N Engl
- Page 183 and 184:
LETTERS3. Guindon S, Gascuel OA. Si
- Page 185 and 186:
BOOKS AND MEDIAin the port cities o
- Page 187 and 188:
ABOUT THE COVERNorth was not intere
- Page 189 and 190:
Earning CME CreditTo obtain credit,
- Page 191:
Emerging Infectious Diseases is a p