- Page 3 and 4: July 2015SynopsisOn the CoverMarian
- Page 5 and 6: 1240 Gastroenteritis OutbreaksCause
- Page 7 and 8: SYNOPSISDisseminated Infections wit
- Page 9 and 10: Disseminated Infections with Talaro
- Page 11 and 12: Disseminated Infections with Talaro
- Page 13 and 14: Macacine Herpesvirus 1 inLong-Taile
- Page 15 and 16: Macacine Herpesvirus 1 in Macaques,
- Page 17 and 18: Macacine Herpesvirus 1 in Macaques,
- Page 19: Macacine Herpesvirus 1 in Macaques,
- Page 23: Malaria among Young Infants, Africa
- Page 26 and 27: RESEARCHFigure 3. Dynamics of 19-kD
- Page 28 and 29: Transdermal Diagnosis of MalariaUsi
- Page 30 and 31: RESEARCHFigure 2. A) Acoustic trace
- Page 32 and 33: RESEARCHof malaria-infected mosquit
- Page 34 and 35: Lack of Transmission amongClose Con
- Page 36 and 37: RESEARCH(IFA) and microneutralizati
- Page 38 and 39: RESEARCHoropharyngeal, and serum sa
- Page 40 and 41: RESEARCH6. Assiri A, McGeer A, Perl
- Page 42 and 43: RESEARCHadvanced genomic sequencing
- Page 44 and 45: RESEARCHTable 2. Next-generation se
- Page 46 and 47: RESEARCHTable 3. Mutation analysis
- Page 48 and 49: RESEARCHReferences1. Baize S, Panne
- Page 50 and 51: Parechovirus Genotype 3 Outbreakamo
- Page 52 and 53: RESEARCHFigure 1. Venn diagramshowi
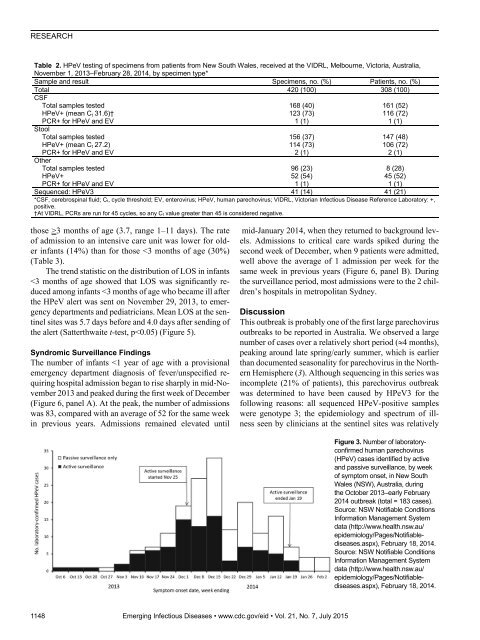
- Page 56 and 57: RESEARCHFigure 5. Distribution of h
- Page 58 and 59: RESEARCHReferences1. Selvarangan R,
- Page 60 and 61: RESEARCHthe left lobe was sampled b
- Page 62 and 63: RESEARCHTable 2. Middle East respir
- Page 64 and 65: RESEARCHseroprevalence in domestic
- Page 66 and 67: RESEARCHmeasure their current surve
- Page 68 and 69: RESEARCHTable 2. States with labora
- Page 70 and 71: RESEARCHFigure 2. Comparison of sur
- Page 72 and 73: RESEARCH9. Centers for Disease Cont
- Page 74 and 75: RESEARCHthe analyses. Cases in pers
- Page 76 and 77: RESEARCHTable 3. Sampling results (
- Page 78 and 79: RESEARCHpresence of Legionella spp.
- Page 80 and 81: Seroprevalence for Hepatitis Eand O
- Page 82 and 83: RESEARCHTable 1. Description of stu
- Page 84 and 85: RESEARCHTable 3. Crude and adjusted
- Page 86 and 87: RESEARCHrates by gender or HIV stat
- Page 88 and 89: RESEARCH25. Taha TE, Kumwenda N, Ka
- Page 90 and 91: POLICY REVIEWDutch Consensus Guidel
- Page 92 and 93: POLICY REVIEWTable 3. Comparison of
- Page 94 and 95: POLICY REVIEW6. Botelho-Nevers E, F
- Page 96 and 97: DISPATCHESFigure 1. Phylogenetic tr
- Page 98 and 99: DISPATCHESSevere Pediatric Adenovir
- Page 100 and 101: DISPATCHESTable 1. Demographics and
- Page 102 and 103: DISPATCHES13. Kim YJ, Hong JY, Lee
- Page 104 and 105:
DISPATCHESTable. Alignment of resid
- Page 106 and 107:
DISPATCHESFigure 2. Interaction of
- Page 108 and 109:
DISPATCHESSchmallenberg Virus Recur
- Page 110 and 111:
DISPATCHESFigure 2. Detection of Sc
- Page 112 and 113:
DISPATCHESFigure 1. Histopathologic
- Page 114:
DISPATCHESFigure 2. Detection of fo
- Page 117 and 118:
Influenza Virus Strains in the Amer
- Page 119 and 120:
Novel Arenavirus Isolates from Nama
- Page 121 and 122:
Novel Arenaviruses, Southern Africa
- Page 123 and 124:
Readability of Ebola Informationon
- Page 125 and 126:
Readability of Ebola Information on
- Page 127 and 128:
Patients under investigation for ME
- Page 129 and 130:
Patients under investigation for ME
- Page 131 and 132:
Wildlife Reservoir for Hepatitis E
- Page 133 and 134:
Asymptomatic Malaria and Other Infe
- Page 135 and 136:
Asymptomatic Malaria in Children fr
- Page 137 and 138:
Bufavirus in Wild Shrews and Nonhum
- Page 139 and 140:
Bufavirus in Wild Shrews and Nonhum
- Page 141 and 142:
Range Expansion for Rat Lungworm in
- Page 143 and 144:
Slow Clearance of Plasmodium falcip
- Page 145 and 146:
Slow Clearance of Plasmodium falcip
- Page 147 and 148:
Gastroenteritis Caused by Norovirus
- Page 149 and 150:
Ebola Virus Stability on Surfaces a
- Page 151 and 152:
Ebola Virus Stability on Surfaces a
- Page 153 and 154:
Outbreak of Ciprofloxacin-Resistant
- Page 155 and 156:
Outbreak of S. sonnei, South KoreaT
- Page 157 and 158:
Rapidly Expanding Range of Highly P
- Page 159 and 160:
Cluster of Ebola Virus Disease, Bon
- Page 161 and 162:
Cluster of Ebola Virus Disease, Lib
- Page 163 and 164:
ANOTHER DIMENSIONThe Past Is Never
- Page 165 and 166:
Measles Epidemic, Boston, Massachus
- Page 167 and 168:
LETTERSInfluenza A(H5N6)Virus Reass
- Page 169 and 170:
LETTERSsystem (8 kb-span paired-end
- Page 171 and 172:
LETTERS3. Van Hong N, Amambua-Ngwa
- Page 173 and 174:
LETTERSTable. Prevalence of Bartone
- Page 175 and 176:
LETTERSavian influenza A(H5N1) viru
- Page 177 and 178:
LETTERSprovinces and a total of 200
- Page 179 and 180:
LETTERS7. Manian FA. Bloodstream in
- Page 181 and 182:
LETTERSforward projections. N Engl
- Page 183 and 184:
LETTERS3. Guindon S, Gascuel OA. Si
- Page 185 and 186:
BOOKS AND MEDIAin the port cities o
- Page 187 and 188:
ABOUT THE COVERNorth was not intere
- Page 189 and 190:
Earning CME CreditTo obtain credit,
- Page 191:
Emerging Infectious Diseases is a p