th - 1988 - 51st ENC Conference
th - 1988 - 51st ENC Conference
th - 1988 - 51st ENC Conference
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
[<br />
~ THE SOURCE OF AN ARTIFACT IN THE IH - IH DECOUPLED<br />
$6 I HETERONUCLEAR CHEMICAL SHIFT CORRELATION EXPERIMENT<br />
Alex D • Bain* , Donald W. Hughes, Dept. of Chemistry, McMaster University,<br />
Hamilton, Ontario, Canada LBS 4MI; and Howard N. Hunter, National<br />
Research Council of Canada, Biotechnology Research Institute, Montreal,<br />
Quebec, Canada H4P 2R2.<br />
When <strong>th</strong>e IH - IH decoupled variation of <strong>th</strong>e heteronuclear<br />
two-dimensional chemical shift correlation experiment (shown below) is<br />
applied to me<strong>th</strong>ylene groups wi<strong>th</strong> inequivalent protons, a strong artifact<br />
may show up at <strong>th</strong>e average chemical shift of <strong>th</strong>e two protons. The<br />
artifact does not appear to be a strong coupling effect, since it appears<br />
in me<strong>th</strong>ylene groups wi<strong>th</strong> large proton chemical shift differences•<br />
However, it behaves apparently erratically wi<strong>th</strong> respect to <strong>th</strong>e shift<br />
difference. Simulations wi<strong>th</strong> <strong>th</strong>e SIMPLTN program indicated a strong<br />
dependence on <strong>th</strong>e delay D2 in <strong>th</strong>e sequence, and <strong>th</strong>is led to a full<br />
explanation. The mechanism for <strong>th</strong>is artifact is presented, along wi<strong>th</strong><br />
experimental confirmation.<br />
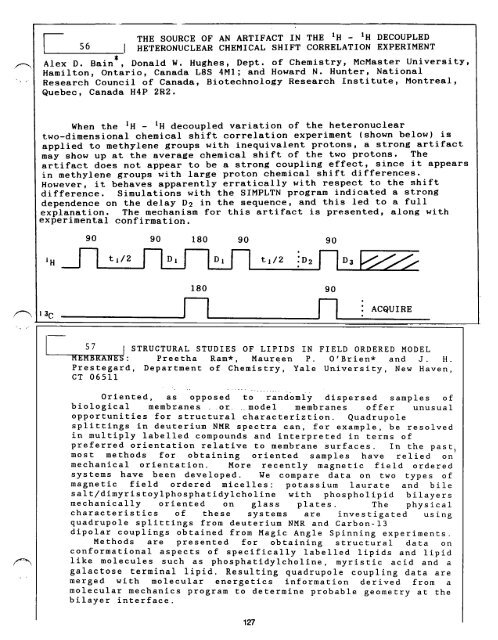
90 90 180 90<br />
,H ~-] t,/2 ~ t,/2<br />
I 3 C<br />
180<br />
V-<br />
90<br />
io2<br />
90<br />
F7<br />
: ACQUIRE<br />
57 I STRUCTURAL STUDIES OF LIPIDS IN FIELD ORDERED MODEL<br />
MEMBRANES: Pree<strong>th</strong>a Ram*, Maureen P. O'Brien* and J. H.<br />
Prestegard, Department of Chemistry, Yale Un.iversity, New Haven,<br />
CT 06511<br />
. . . . . . . . . . . °<br />
Oriented, as opposed to randomly dispersed samples of<br />
biological membranes . o~ model membranes offer unusual<br />
opportunities for structural characteriztion. Quadrupole<br />
splittings in deuterium NMR spectra can, for example, be resolved<br />
in multiply labelled compounds and interpreted in terms of<br />
preferred orientation relative to membrane surfaces. In <strong>th</strong>e past<br />
most me<strong>th</strong>ods for obtaining oriented samples have relied on<br />
mechanical orientation. More recently magnetic field ordered<br />
systems have been developed. We compare data on two types of<br />
magnetic field ordered micelles: potassium laurate and bile<br />
salt/dimyristoylphosphatidylcholine wi<strong>th</strong> phospholipid bilayers<br />
mechanically oriented on glass plates. The physical<br />
characteristics of <strong>th</strong>ese systems are investigated using<br />
quadrupole splittings from deuterium NMR and Carbon-13<br />
dipolar couplings obtained from Magic Angle Spinning experiments.<br />
Me<strong>th</strong>ods are presented for obtaining structural data on<br />
conformational aspects of specifically labelled lipids and lipid<br />
like molecules such as phosphatidylcholine, myristic acid and a<br />
galactose terminal lipid. Resulting quadrupole coupling data are<br />
merged wi<strong>th</strong> molecular energetics information derived from a<br />
molecular mechanics program to determine probable geometry at <strong>th</strong>e<br />
bilayer interface.<br />
127