Electronic Material Properties - und Geowissenschaften ...
Electronic Material Properties - und Geowissenschaften ...
Electronic Material Properties - und Geowissenschaften ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Synchrotron Induced Photoelectron Spectroscopy at the Solid-<br />
Liquid Interface of Dye Sensitized Solar Cells<br />
Konrad Schwanitz, Eric Mankel, Ralf Hunger, Thomas Mayer,<br />
and Wolfram Jaegermann<br />
At BESSY we run the experimental station SoLiAS, dedicated to solid-liquid interface<br />
analysis. SoLiAS allows for the transfer of wet chemically prepared surfaces to the ultra<br />
high vacuum without contact to ambient air. In addition in situ (co)adsorption of volatile<br />
solvent species onto liquid nitrogen cooled samples is possible. SoLiAS proves to be very<br />
useful in analyzing the chemical and electronic structure of solid-liquid interfaces e.g. of<br />
dye sensitized solar cells. A monolayer of Ru(N3)-dye was adsorbed from ethanol solution<br />
<strong>und</strong>er clean N2 atmosphere in an UHV-integrated electrochemical cell (EC). Acetonitrile or<br />
benzene were adsorbed from the liquid in the EC or in situ from the gas phase. Ex situ<br />
sintered nanocrystalline anatase substrates as well as in situ deposited polycrystalline<br />
TiO2 samples were used. Distinct reversible changes occur in synchrotron induced<br />
photoelectron valence band and core level spectra when the solvent is adsorbed to<br />
pristine and dye covered TiO2 substrates. Based on the experimental results the alignment<br />
of electronic states and a model on the dye-solvent interaction have been deduced.<br />
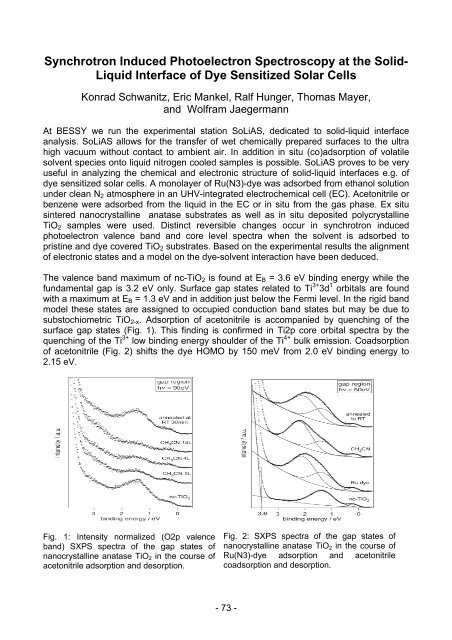
The valence band maximum of nc-TiO2 is fo<strong>und</strong> at EB = 3.6 eV binding energy while the<br />
f<strong>und</strong>amental gap is 3.2 eV only. Surface gap states related to Ti 3+ 3d 1 orbitals are fo<strong>und</strong><br />
with a maximum at EB = 1.3 eV and in addition just below the Fermi level. In the rigid band<br />
model these states are assigned to occupied conduction band states but may be due to<br />
substochiometric TiO2-x. Adsorption of acetonitrile is accompanied by quenching of the<br />
surface gap states (Fig. 1). This finding is confirmed in Ti2p core orbital spectra by the<br />
quenching of the Ti 3+ low binding energy shoulder of the Ti 4+ bulk emission. Coadsorption<br />
of acetonitrile (Fig. 2) shifts the dye HOMO by 150 meV from 2.0 eV binding energy to<br />
2.15 eV.<br />
Fig. 1: Intensity normalized (O2p valence<br />
band) SXPS spectra of the gap states of<br />
nanocrystalline anatase TiO2 in the course of<br />
acetonitrile adsorption and desorption.<br />
Fig. 2: SXPS spectra of the gap states of<br />
nanocrystalline anatase TiO2 in the course of<br />
Ru(N3)-dye adsorption and acetonitrile<br />
coadsorption and desorption.<br />
- 73 -