Electronic Material Properties - und Geowissenschaften ...
Electronic Material Properties - und Geowissenschaften ...
Electronic Material Properties - und Geowissenschaften ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
The spectrum shows that apart from C, hydrogen has been implanted. In the plasma,<br />
different hydrogen-containing ionic species are present, such as CH4 + , CH3 + , and<br />
CH2 + . The H profile starts at a certain level at the surface, and drops almost an order<br />
of magnitude in depth. The carbon profiles of samples, treated with different process<br />
times, are compared in Fig. 2. They were extracted from the C-12 signal. A strict<br />
quantitative comparison is not possible, as the secondary ion yield might slightly<br />
differ, however, one can state that the amount of incorporated carbon increases with<br />
process time. Also, the shape of the depth profiles change. At the lowest process<br />
time, the profile drops monotonely with depth.<br />
A process time of 1 h leads to a typical PIII implantation profile: a slight drop below<br />
the surface, followed by an increase, and a slope down to the backgro<strong>und</strong> level.<br />
When the process time is doubled, the profile changes further. A plateau seems to be<br />
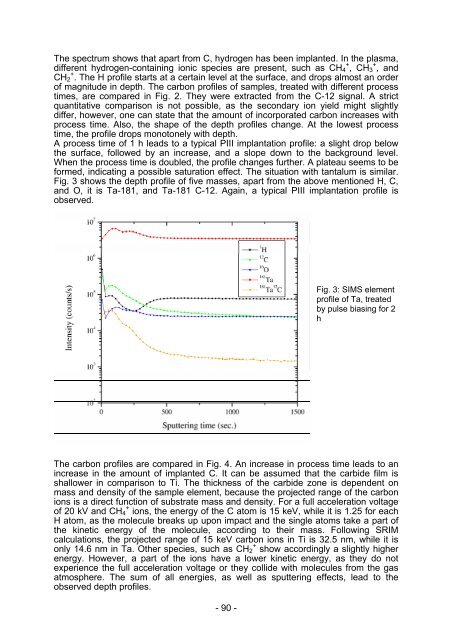
formed, indicating a possible saturation effect. The situation with tantalum is similar.<br />
Fig. 3 shows the depth profile of five masses, apart from the above mentioned H, C,<br />
and O, it is Ta-181, and Ta-181 C-12. Again, a typical PIII implantation profile is<br />
observed.<br />
Fig. 3: SIMS element<br />
profile of Ta, treated<br />
by pulse biasing for 2<br />
h<br />
The carbon profiles are compared in Fig. 4. An increase in process time leads to an<br />
increase in the amount of implanted C. It can be assumed that the carbide film is<br />
shallower in comparison to Ti. The thickness of the carbide zone is dependent on<br />
mass and density of the sample element, because the projected range of the carbon<br />
ions is a direct function of substrate mass and density. For a full acceleration voltage<br />
of 20 kV and CH4 + ions, the energy of the C atom is 15 keV, while it is 1.25 for each<br />
H atom, as the molecule breaks up upon impact and the single atoms take a part of<br />
the kinetic energy of the molecule, according to their mass. Following SRIM<br />
calculations, the projected range of 15 keV carbon ions in Ti is 32.5 nm, while it is<br />
only 14.6 nm in Ta. Other species, such as CH2 + show accordingly a slightly higher<br />
energy. However, a part of the ions have a lower kinetic energy, as they do not<br />
experience the full acceleration voltage or they collide with molecules from the gas<br />
atmosphere. The sum of all energies, as well as sputtering effects, lead to the<br />
observed depth profiles.<br />
- 90 -