research activities in 2007 - CSEM
research activities in 2007 - CSEM
research activities in 2007 - CSEM
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Cl<strong>in</strong>ical Validation Results of the Long-Term Medical Survey System<br />
O. Chételat, A. O'Hare, P. Pilloud, J-M. Koller, S. Droz, A. Ridolfi, P. Theurillat, P. Renevey, J. Solà I Caros, O. Grossenbacher<br />
The paper describes the cl<strong>in</strong>ical validation of a physiological multi-parameter monitor<strong>in</strong>g system made by <strong>CSEM</strong> for ESA. Results of the validation<br />
showed that the system fulfils its purpose. The real conditions of the validation also h<strong>in</strong>ted at further potential enhancements.<br />
The European Space Agency (ESA) commissioned <strong>CSEM</strong> to<br />
design, build, validate and deliver one fully operational ground<br />
prototype of a system (LTMS2) measur<strong>in</strong>g physiological<br />
parameters. The system was shipped and will be used dur<strong>in</strong>g<br />
2008 at Concordia station (www.concordiastation.com) <strong>in</strong><br />
Antarctica to study the physiological adaptation of manned<br />
crews to remote, isolated and extreme environments. The<br />
underly<strong>in</strong>g long-term objective for ESA is to obta<strong>in</strong> experience<br />
<strong>in</strong> the field which will be used <strong>in</strong> prepar<strong>in</strong>g a possible manned<br />
mission to Mars around the year 2030. This paper describes<br />
the cl<strong>in</strong>ical validation of the system performed <strong>in</strong> December<br />
<strong>2007</strong> on volunteers at the Emergency Care Unit of the<br />
University Hospital of Bern (Inselspital).<br />
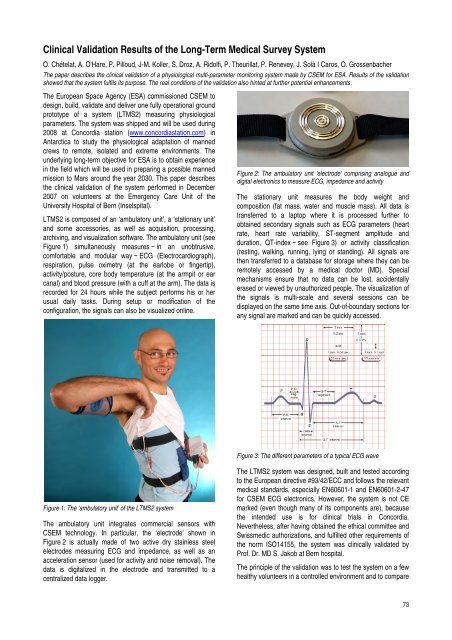
LTMS2 is composed of an ‘ambulatory unit’, a ‘stationary unit’<br />
and some accessories, as well as acquisition, process<strong>in</strong>g,<br />
archiv<strong>in</strong>g, and visualization software. The ambulatory unit (see<br />
Figure 1) simultaneously measures − <strong>in</strong> an unobtrusive,<br />
comfortable and modular way − ECG (Electrocardiograph),<br />
respiration, pulse oximetry (at the earlobe or f<strong>in</strong>gertip),<br />
activity/posture, core body temperature (at the armpit or ear<br />
canal) and blood pressure (with a cuff at the arm). The data is<br />
recorded for 24 hours while the subject performs his or her<br />
usual daily tasks. Dur<strong>in</strong>g setup or modification of the<br />
configuration, the signals can also be visualized onl<strong>in</strong>e.<br />
Figure 1: The ‘ambulatory unit’ of the LTMS2 system<br />
The ambulatory unit <strong>in</strong>tegrates commercial sensors with<br />
<strong>CSEM</strong> technology. In particular, the ‘electrode’ shown <strong>in</strong><br />
Figure 2 is actually made of two active dry sta<strong>in</strong>less steel<br />
electrodes measur<strong>in</strong>g ECG and impedance, as well as an<br />
acceleration sensor (used for activity and noise removal). The<br />
data is digitalized <strong>in</strong> the electrode and transmitted to a<br />
centralized data logger.<br />
Figure 2: The ambulatory unit ‘electrode’ compris<strong>in</strong>g analogue and<br />
digital electronics to measure ECG, impedance and activity<br />
The stationary unit measures the body weight and<br />
composition (fat mass, water and muscle mass). All data is<br />
transferred to a laptop where it is processed further to<br />
obta<strong>in</strong>ed secondary signals such as ECG parameters (heart<br />
rate, heart rate variability, ST-segment amplitude and<br />
duration, QT-<strong>in</strong>dex − see Figure 3) or activity classification<br />
(rest<strong>in</strong>g, walk<strong>in</strong>g, runn<strong>in</strong>g, ly<strong>in</strong>g or stand<strong>in</strong>g). All signals are<br />
then transferred to a database for storage where they can be<br />
remotely accessed by a medical doctor (MD). Special<br />
mechanisms ensure that no data can be lost, accidentally<br />
erased or viewed by unauthorized people. The visualization of<br />
the signals is multi-scale and several sessions can be<br />
displayed on the same time axis. Out-of-boundary sections for<br />
any signal are marked and can be quickly accessed.<br />
Figure 3: The different parameters of a typical ECG wave<br />
The LTMS2 system was designed, built and tested accord<strong>in</strong>g<br />
to the European directive #93/42/ECC and follows the relevant<br />
medical standards, especially EN60601-1 and EN60601-2-47<br />
for <strong>CSEM</strong> ECG electronics. However, the system is not CE<br />
marked (even though many of its components are), because<br />
the <strong>in</strong>tended use is for cl<strong>in</strong>ical trials <strong>in</strong> Concordia.<br />
Nevertheless, after hav<strong>in</strong>g obta<strong>in</strong>ed the ethical committee and<br />
Swissmedic authorizations, and fulfilled other requirements of<br />
the norm ISO14155, the system was cl<strong>in</strong>ically validated by<br />
Prof. Dr. MD S. Jakob at Bern hospital.<br />
The pr<strong>in</strong>ciple of the validation was to test the system on a few<br />
healthy volunteers <strong>in</strong> a controlled environment and to compare<br />
73