AstraZeneca Annual Report and Form 20-F Information 2011
AstraZeneca Annual Report and Form 20-F Information 2011
AstraZeneca Annual Report and Form 20-F Information 2011
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
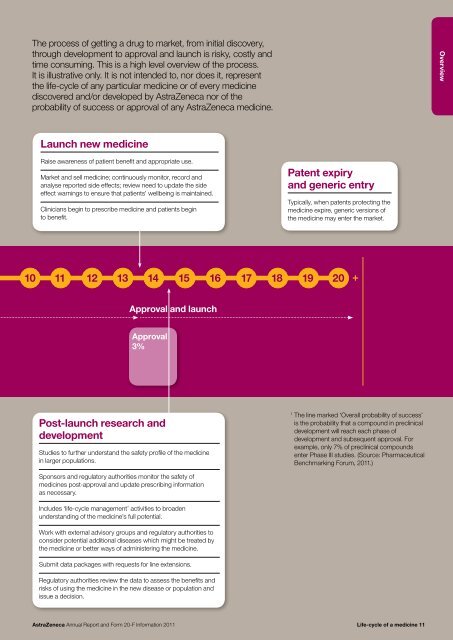
The process of getting a drug to market, from initial discovery,through development to approval <strong>and</strong> launch is risky, costly <strong>and</strong>time consuming. This is a high level overview of the process.It is illustrative only. It is not intended to, nor does it, representthe life-cycle of any particular medicine or of every medicinediscovered <strong>and</strong>/or developed by <strong>AstraZeneca</strong> nor of theprobability of success or approval of any <strong>AstraZeneca</strong> medicine.OverviewLaunch new medicineRaise awareness of patient benefit <strong>and</strong> appropriate use.Market <strong>and</strong> sell medicine; continuously monitor, record <strong>and</strong>analyse reported side effects; review need to update the sideeffect warnings to ensure that patients’ wellbeing is maintained.Clinicians begin to prescribe medicine <strong>and</strong> patients beginto benefit.Patent expiry<strong>and</strong> generic entryTypically, when patents protecting themedicine expire, generic versions ofthe medicine may enter the market.10 11 12 13 14 15 16 17 18 19 <strong>20</strong>+Approval <strong>and</strong> launchApproval3%Post-launch research <strong>and</strong>developmentStudies to further underst<strong>and</strong> the safety profile of the medicinein larger populations.1The line marked ‘Overall probability of success’is the probability that a compound in preclinicaldevelopment will reach each phase ofdevelopment <strong>and</strong> subsequent approval. Forexample, only 7% of preclinical compoundsenter Phase III studies. (Source: PharmaceuticalBenchmarking Forum, <strong>20</strong>11.)Sponsors <strong>and</strong> regulatory authorities monitor the safety ofmedicines post-approval <strong>and</strong> update prescribing informationas necessary.Includes ‘life-cycle management’ activities to broadenunderst<strong>and</strong>ing of the medicine’s full potential.Work with external advisory groups <strong>and</strong> regulatory authorities toconsider potential additional diseases which might be treated bythe medicine or better ways of administering the medicine.Submit data packages with requests for line extensions.Regulatory authorities review the data to assess the benefits <strong>and</strong>risks of using the medicine in the new disease or population <strong>and</strong>issue a decision.<strong>AstraZeneca</strong> <strong>Annual</strong> <strong>Report</strong> <strong>and</strong> <strong>Form</strong> <strong>20</strong>-F <strong>Information</strong> <strong>20</strong>11 Life-cycle of a medicine 11