AstraZeneca Annual Report and Form 20-F Information 2011
AstraZeneca Annual Report and Form 20-F Information 2011
AstraZeneca Annual Report and Form 20-F Information 2011
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
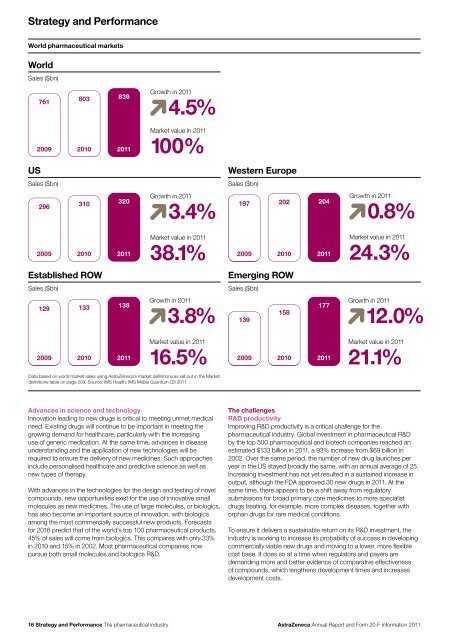
Strategy <strong>and</strong> PerformanceWorld pharmaceutical marketsWorld Sales($bn)Sales ($bn)761803839Growth in <strong>20</strong>114.5%<strong>20</strong>09<strong>20</strong>10 <strong>20</strong>11Market value in <strong>20</strong>11100%USSales($bn)Sales ($bn)Western Sales Europe($bn)Sales ($bn)2963103<strong>20</strong>Growth in <strong>20</strong>113.4%197<strong>20</strong>2<strong>20</strong>4Growth in <strong>20</strong>110.8%<strong>20</strong>09<strong>20</strong>10 <strong>20</strong>11Market value in <strong>20</strong>1138.1%<strong>20</strong>09<strong>20</strong>10 <strong>20</strong>11Market value in <strong>20</strong>1124.3%EstablishedSalesROW($bn)Sales ($bn)EmergingSalesROW($bn)Sales ($bn)129133138Growth in <strong>20</strong>113.8%139158177Growth in <strong>20</strong>1112.0%<strong>20</strong>09<strong>20</strong>10 <strong>20</strong>11Market value in <strong>20</strong>1116.5%<strong>20</strong>09<strong>20</strong>10 <strong>20</strong>11Market value in <strong>20</strong>1121.1%Data based on world market sales using <strong>AstraZeneca</strong>’s market definitions as set out in the Marketdefinitions table on page <strong>20</strong>9. Source: IMS Health, IMS Midas Quantum Q3 <strong>20</strong>11.Advances in science <strong>and</strong> technologyInnovation leading to new drugs is critical to meeting unmet medicalneed. Existing drugs will continue to be important in meeting thegrowing dem<strong>and</strong> for healthcare, particularly with the increasinguse of generic medication. At the same time, advances in diseaseunderst<strong>and</strong>ing <strong>and</strong> the application of new technologies will berequired to ensure the delivery of new medicines. Such approachesinclude personalised healthcare <strong>and</strong> predictive science as well asnew types of therapy.With advances in the technologies for the design <strong>and</strong> testing of novelcompounds, new opportunities exist for the use of innovative smallmolecules as new medicines. The use of large molecules, or biologics,has also become an important source of innovation, with biologicsamong the most commercially successful new products. Forecastsfor <strong>20</strong>16 predict that of the world’s top 100 pharmaceutical products,45% of sales will come from biologics. This compares with only 33%in <strong>20</strong>10 <strong>and</strong> 15% in <strong>20</strong>02. Most pharmaceutical companies nowpursue both small molecules <strong>and</strong> biologics R&D.The challengesR&D productivityImproving R&D productivity is a critical challenge for thepharmaceutical industry. Global investment in pharmaceutical R&Dby the top 500 pharmaceutical <strong>and</strong> biotech companies reached anestimated $133 billion in <strong>20</strong>11, a 93% increase from $69 billion in<strong>20</strong>02. Over the same period, the number of new drug launches peryear in the US stayed broadly the same, with an annual average of 25.Increasing investment has not yet resulted in a sustained increase inoutput, although the FDA approved 30 new drugs in <strong>20</strong>11. At thesame time, there appears to be a shift away from regulatorysubmissions for broad primary care medicines to more specialistdrugs treating, for example, more complex diseases, together withorphan drugs for rare medical conditions.To ensure it delivers a sustainable return on its R&D investment, theindustry is working to increase its probability of success in developingcommercially viable new drugs <strong>and</strong> moving to a lower, more flexiblecost base. It does so at a time when regulators <strong>and</strong> payers aredem<strong>and</strong>ing more <strong>and</strong> better evidence of comparative effectivenessof compounds, which lengthens development times <strong>and</strong> increasesdevelopment costs.16 Strategy <strong>and</strong> Performance The pharmaceutical industry<strong>AstraZeneca</strong> <strong>Annual</strong> <strong>Report</strong> <strong>and</strong> <strong>Form</strong> <strong>20</strong>-F <strong>Information</strong> <strong>20</strong>11