AstraZeneca Annual Report and Form 20-F Information 2011
AstraZeneca Annual Report and Form 20-F Information 2011
AstraZeneca Annual Report and Form 20-F Information 2011
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
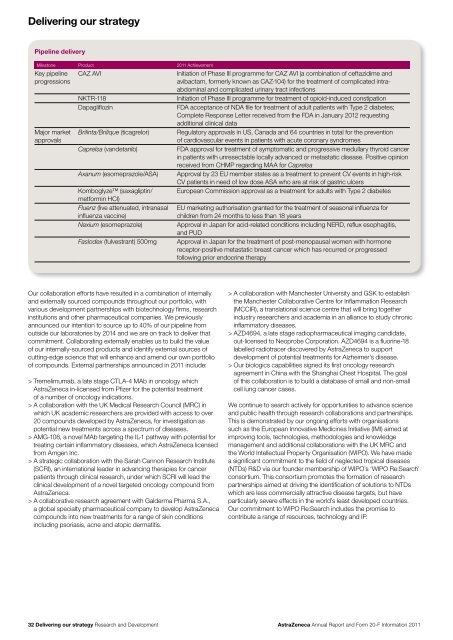
Delivering our strategyPipeline deliveryMilestone Product <strong>20</strong>11 AchievementKey pipelineprogressionsMajor marketapprovalsCAZ AVINKTR-118DapagliflozinBrilinta/Brilique (ticagrelor)Caprelsa (v<strong>and</strong>etanib)Axanum (esomeprazole/ASA)Komboglyze (saxagliptin/metformin HCI)Fluenz (live attenuated, intranasalinfluenza vaccine)Nexium (esomeprazole)Faslodex (fulvestrant) 500mgInitiation of Phase III programme for CAZ AVI (a combination of ceftazidime <strong>and</strong>avibactam, formerly known as CAZ-104) for the treatment of complicated intraabdominal<strong>and</strong> complicated urinary tract infectionsInitiation of Phase III programme for treatment of opioid-induced constipationFDA acceptance of NDA file for treatment of adult patients with Type 2 diabetes;Complete Response Letter received from the FDA in January <strong>20</strong>12 requestingadditional clinical dataRegulatory approvals in US, Canada <strong>and</strong> 64 countries in total for the preventionof cardiovascular events in patients with acute coronary syndromesFDA approval for treatment of symptomatic <strong>and</strong> progressive medullary thyroid cancerin patients with unresectable locally advanced or metastatic disease. Positive opinionreceived from CHMP regarding MAA for CaprelsaApproval by 23 EU member states as a treatment to prevent CV events in high-riskCV patients in need of low dose ASA who are at risk of gastric ulcersEuropean Commission approval as a treatment for adults with Type 2 diabetesEU marketing authorisation granted for the treatment of seasonal influenza forchildren from 24 months to less than 18 yearsApproval in Japan for acid-related conditions including NERD, reflux esophagitis,<strong>and</strong> PUDApproval in Japan for the treatment of post-menopausal women with hormonereceptor-positive metastatic breast cancer which has recurred or progressedfollowing prior endocrine therapyOur collaboration efforts have resulted in a combination of internally<strong>and</strong> externally sourced compounds throughout our portfolio, withvarious development partnerships with biotechnology firms, researchinstitutions <strong>and</strong> other pharmaceutical companies. We previouslyannounced our intention to source up to 40% of our pipeline fromoutside our laboratories by <strong>20</strong>14 <strong>and</strong> we are on track to deliver thatcommitment. Collaborating externally enables us to build the valueof our internally-sourced products <strong>and</strong> identify external sources ofcutting-edge science that will enhance <strong>and</strong> amend our own portfolioof compounds. External partnerships announced in <strong>20</strong>11 include:> Tremelimumab, a late stage CTLA-4 MAb in oncology which<strong>AstraZeneca</strong> in-licensed from Pfizer for the potential treatmentof a number of oncology indications.> A collaboration with the UK Medical Research Council (MRC) inwhich UK academic researchers are provided with access to over<strong>20</strong> compounds developed by <strong>AstraZeneca</strong>, for investigation aspotential new treatments across a spectrum of diseases.> AMG-108, a novel MAb targeting the IL-1 pathway with potential fortreating certain inflammatory diseases, which <strong>AstraZeneca</strong> licensedfrom Amgen Inc.> A strategic collaboration with the Sarah Cannon Research Institute(SCRI), an international leader in advancing therapies for cancerpatients through clinical research, under which SCRI will lead theclinical development of a novel targeted oncology compound from<strong>AstraZeneca</strong>.> A collaborative research agreement with Galderma Pharma S.A.,a global specialty pharmaceutical company to develop <strong>AstraZeneca</strong>compounds into new treatments for a range of skin conditionsincluding psoriasis, acne <strong>and</strong> atopic dermatitis.> A collaboration with Manchester University <strong>and</strong> GSK to establishthe Manchester Collaborative Centre for Inflammation Research(MCCIR), a translational science centre that will bring togetherindustry researchers <strong>and</strong> academia in an alliance to study chronicinflammatory diseases.> AZD4694, a late stage radiopharmaceutical imaging c<strong>and</strong>idate,out-licensed to Neoprobe Corporation. AZD4694 is a fluorine-18labelled radiotracer discovered by <strong>AstraZeneca</strong> to supportdevelopment of potential treatments for Alzheimer’s disease.> Our biologics capabilities signed its first oncology researchagreement in China with the Shanghai Chest Hospital. The goalof this collaboration is to build a database of small <strong>and</strong> non-smallcell lung cancer cases.We continue to search actively for opportunities to advance science<strong>and</strong> public health through research collaborations <strong>and</strong> partnerships.This is demonstrated by our ongoing efforts with organisationssuch as the European Innovative Medicines Initiative (IMI) aimed atimproving tools, technologies, methodologies <strong>and</strong> knowledgemanagement <strong>and</strong> additional collaborations with the UK MRC <strong>and</strong>the World Intellectual Property Organisation (WIPO). We have madea significant commitment to the field of neglected tropical diseases(NTDs) R&D via our founder membership of WIPO’s ‘WIPO Re:Search’consortium. This consortium promotes the formation of researchpartnerships aimed at driving the identification of solutions to NTDswhich are less commercially attractive disease targets, but haveparticularly severe effects in the world’s least developed countries.Our commitment to WIPO Re:Search includes the promise tocontribute a range of resources, technology <strong>and</strong> IP.32 Delivering our strategy Research <strong>and</strong> Development<strong>AstraZeneca</strong> <strong>Annual</strong> <strong>Report</strong> <strong>and</strong> <strong>Form</strong> <strong>20</strong>-F <strong>Information</strong> <strong>20</strong>11