AstraZeneca Annual Report and Form 20-F Information 2011
AstraZeneca Annual Report and Form 20-F Information 2011
AstraZeneca Annual Report and Form 20-F Information 2011
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
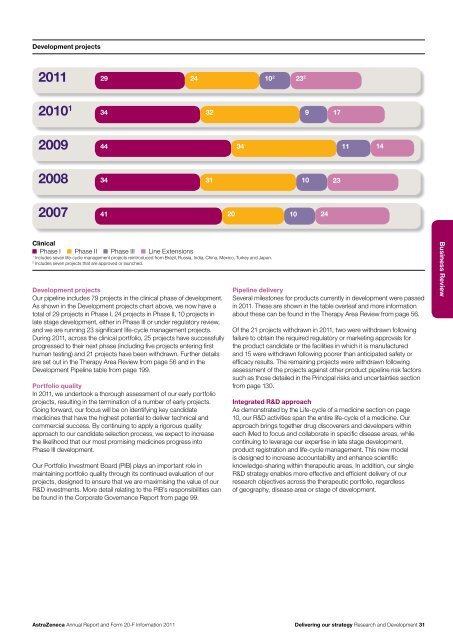
Development projects<strong>20</strong>11<strong>20</strong>10 12934243210 2 23 29 17<strong>20</strong>0944 34 11 14<strong>20</strong>0834 31 10 23<strong>20</strong>0741 <strong>20</strong> 10 24ClinicalPhase I Phase II Phase III Line Extensions1Includes seven life-cycle management projects reintroduced from Brazil, Russia, India, China, Mexico, Turkey <strong>and</strong> Japan.2Includes seven projects that are approved or launched.Development projectsOur pipeline includes 79 projects in the clinical phase of development.As shown in the Development projects chart above, we now have atotal of 29 projects in Phase I, 24 projects in Phase II, 10 projects inlate stage development, either in Phase III or under regulatory review,<strong>and</strong> we are running 23 significant life-cycle management projects.During <strong>20</strong>11, across the clinical portfolio, 25 projects have successfullyprogressed to their next phase (including five projects entering firsthuman testing) <strong>and</strong> 21 projects have been withdrawn. Further detailsare set out in the Therapy Area Review from page 56 <strong>and</strong> in theDevelopment Pipeline table from page 199.Portfolio qualityIn <strong>20</strong>11, we undertook a thorough assessment of our early portfolioprojects, resulting in the termination of a number of early projects.Going forward, our focus will be on identifying key c<strong>and</strong>idatemedicines that have the highest potential to deliver technical <strong>and</strong>commercial success. By continuing to apply a rigorous qualityapproach to our c<strong>and</strong>idate selection process, we expect to increasethe likelihood that our most promising medicines progress intoPhase III development.Our Portfolio Investment Board (PIB) plays an important role inmaintaining portfolio quality through its continued evaluation of ourprojects, designed to ensure that we are maximising the value of ourR&D investments. More detail relating to the PIB’s responsibilities canbe found in the Corporate Governance <strong>Report</strong> from page 99.Pipeline deliverySeveral milestones for products currently in development were passedin <strong>20</strong>11. These are shown in the table overleaf <strong>and</strong> more informationabout these can be found in the Therapy Area Review from page 56.Of the 21 projects withdrawn in <strong>20</strong>11, two were withdrawn followingfailure to obtain the required regulatory or marketing approvals forthe product c<strong>and</strong>idate or the facilities in which it is manufactured<strong>and</strong> 15 were withdrawn following poorer than anticipated safety orefficacy results. The remaining projects were withdrawn followingassessment of the projects against other product pipeline risk factorssuch as those detailed in the Principal risks <strong>and</strong> uncertainties sectionfrom page 130.Integrated R&D approachAs demonstrated by the Life-cycle of a medicine section on page10, our R&D activities span the entire life-cycle of a medicine. Ourapproach brings together drug discoverers <strong>and</strong> developers withineach iMed to focus <strong>and</strong> collaborate in specific disease areas, whilecontinuing to leverage our expertise in late stage development,product registration <strong>and</strong> life-cycle management. This new modelis designed to increase accountability <strong>and</strong> enhance scientificknowledge-sharing within therapeutic areas. In addition, our singleR&D strategy enables more effective <strong>and</strong> efficient delivery of ourresearch objectives across the therapeutic portfolio, regardlessof geography, disease area or stage of development.Business Review<strong>AstraZeneca</strong> <strong>Annual</strong> <strong>Report</strong> <strong>and</strong> <strong>Form</strong> <strong>20</strong>-F <strong>Information</strong> <strong>20</strong>11Delivering our strategy Research <strong>and</strong> Development 31