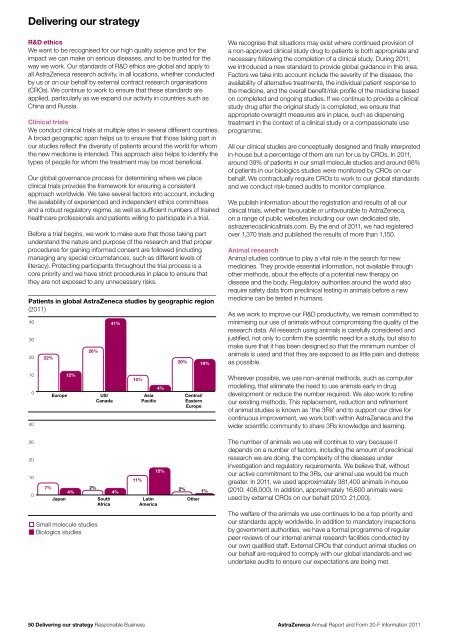
Delivering our strategyR&D ethicsWe want to be recognised for our high quality science <strong>and</strong> for theimpact we can make on serious diseases, <strong>and</strong> to be trusted for theway we work. Our st<strong>and</strong>ards of R&D ethics are global <strong>and</strong> apply toall <strong>AstraZeneca</strong> research activity, in all locations, whether conductedby us or on our behalf by external contract research organisations(CROs). We continue to work to ensure that these st<strong>and</strong>ards areapplied, particularly as we exp<strong>and</strong> our activity in countries such asChina <strong>and</strong> Russia.Clinical trialsWe conduct clinical trials at multiple sites in several different countries.A broad geographic span helps us to ensure that those taking part inour studies reflect the diversity of patients around the world for whomthe new medicine is intended. This approach also helps to identify thetypes of people for whom the treatment may be most beneficial.Our global governance process for determining where we placeclinical trials provides the framework for ensuring a consistentapproach worldwide. We take several factors into account, includingthe availability of experienced <strong>and</strong> independent ethics committees<strong>and</strong> a robust regulatory regime, as well as sufficient numbers of trainedhealthcare professionals <strong>and</strong> patients willing to participate in a trial.Before a trial begins, we work to make sure that those taking partunderst<strong>and</strong> the nature <strong>and</strong> purpose of the research <strong>and</strong> that properprocedures for gaining informed consent are followed (includingmanaging any special circumstances, such as different levels ofliteracy). Protecting participants throughout the trial process is acore priority <strong>and</strong> we have strict procedures in place to ensure thatthey are not exposed to any unnecessary risks.Patients in global <strong>AstraZeneca</strong> studies by geographic region(<strong>20</strong>11)4030<strong>20</strong>22%26%41%<strong>20</strong>%19%We recognise that situations may exist where continued provision ofa non-approved clinical study drug to patients is both appropriate <strong>and</strong>necessary following the completion of a clinical study. During <strong>20</strong>11,we introduced a new st<strong>and</strong>ard to provide global guidance in this area.Factors we take into account include the severity of the disease, theavailability of alternative treatments, the individual patient response tothe medicine, <strong>and</strong> the overall benefit/risk profile of the medicine basedon completed <strong>and</strong> ongoing studies. If we continue to provide a clinicalstudy drug after the original study is completed, we ensure thatappropriate oversight measures are in place, such as dispensingtreatment in the context of a clinical study or a compassionate useprogramme.All our clinical studies are conceptually designed <strong>and</strong> finally interpretedin-house but a percentage of them are run for us by CROs. In <strong>20</strong>11,around 39% of patients in our small molecule studies <strong>and</strong> around 66%of patients in our biologics studies were monitored by CROs on ourbehalf. We contractually require CROs to work to our global st<strong>and</strong>ards<strong>and</strong> we conduct risk-based audits to monitor compliance.We publish information about the registration <strong>and</strong> results of all ourclinical trials, whether favourable or unfavourable to <strong>AstraZeneca</strong>,on a range of public websites including our own dedicated site,astrazenecaclinicaltrials.com. By the end of <strong>20</strong>11, we had registeredover 1,370 trials <strong>and</strong> published the results of more than 1,150.Animal researchAnimal studies continue to play a vital role in the search for newmedicines. They provide essential information, not available throughother methods, about the effects of a potential new therapy ondisease <strong>and</strong> the body. Regulatory authorities around the world alsorequire safety data from preclinical testing in animals before a newmedicine can be tested in humans.As we work to improve our R&D productivity, we remain committed tominimising our use of animals without compromising the quality of theresearch data. All research using animals is carefully considered <strong>and</strong>justified, not only to confirm the scientific need for a study, but also tomake sure that it has been designed so that the minimum number ofanimals is used <strong>and</strong> that they are exposed to as little pain <strong>and</strong> distressas possible.10040Europe12%US/Canada10%AsiaPacific4%Central/EasternEuropeWherever possible, we use non-animal methods, such as computermodelling, that eliminate the need to use animals early in drugdevelopment or reduce the number required. We also work to refineour existing methods. This replacement, reduction <strong>and</strong> refinementof animal studies is known as ‘the 3Rs’ <strong>and</strong> to support our drive forcontinuous improvement, we work both within <strong>AstraZeneca</strong> <strong>and</strong> thewider scientific community to share 3Rs knowledge <strong>and</strong> learning.30<strong>20</strong>1007%Japan4%2%Small molecule studiesBiologics studiesSouthAfrica11%2%4% 1%LatinAmerica15%OtherThe number of animals we use will continue to vary because itdepends on a number of factors, including the amount of preclinicalresearch we are doing, the complexity of the diseases underinvestigation <strong>and</strong> regulatory requirements. We believe that, withoutour active commitment to the 3Rs, our animal use would be muchgreater. In <strong>20</strong>11, we used approximately 381,400 animals in-house(<strong>20</strong>10: 408,000). In addition, approximately 16,600 animals wereused by external CROs on our behalf (<strong>20</strong>10: 21,000).The welfare of the animals we use continues to be a top priority <strong>and</strong>our st<strong>and</strong>ards apply worldwide. In addition to m<strong>and</strong>atory inspectionsby government authorities, we have a formal programme of regularpeer reviews of our internal animal research facilities conducted byour own qualified staff. External CROs that conduct animal studies onour behalf are required to comply with our global st<strong>and</strong>ards <strong>and</strong> weundertake audits to ensure our expectations are being met.50 Delivering our strategy Responsible Business<strong>AstraZeneca</strong> <strong>Annual</strong> <strong>Report</strong> <strong>and</strong> <strong>Form</strong> <strong>20</strong>-F <strong>Information</strong> <strong>20</strong>11
Protests<strong>AstraZeneca</strong> acknowledges the right of every individual to expresstheir views on the use of animals in research but we condemn theuse of violence <strong>and</strong> other illegal acts. We firmly reject any harassment,intimidation or harming of our employees <strong>and</strong> their families, oursuppliers <strong>and</strong> our other stakeholders as totally unacceptable.Global KPI: Breaches of external sales <strong>and</strong> marketing codes<strong>and</strong> regulations ruled by external bodies24Sales <strong>and</strong> marketing ethicsDelivering consistently high st<strong>and</strong>ards of sales <strong>and</strong> marketing practicecontinues to be one of our top priorities <strong>and</strong> is at the core of ourcommitment to driving commercial success responsibly. Our activitiescentre on ensuring that the appropriate information is provided tothose who need it to support the safe <strong>and</strong> effective use of ourmedicines <strong>and</strong> enhance patient care.<strong>20</strong>091117<strong>20</strong>10 <strong>20</strong>11We have always had a range of sales <strong>and</strong> marketing policies <strong>and</strong>st<strong>and</strong>ards in place but, following a review in <strong>20</strong>10, we furtherstrengthened the requirements <strong>and</strong> consolidated the range to forma single new Global Policy on External Interactions (the Policy).Launched in April <strong>20</strong>11, the new Policy provides a single common,principle-based approach to all our interactions worldwide. Everyonein <strong>AstraZeneca</strong>, wherever they are located, is required to work to ourglobal st<strong>and</strong>ards of ethical sales <strong>and</strong> marketing practice. We believethis is especially important as we grow our business in EmergingMarkets, such as China <strong>and</strong> Russia, alongside our continued effortsin Established Markets, including the US <strong>and</strong> Japan.The diversity of business cultures around the world means thatputting a global approach into practice at a local level is a challenge.Nevertheless, we are committed to making it work. During <strong>20</strong>11,we continued to provide targeted training for our people to ensureexpectations <strong>and</strong> accountabilities were clear <strong>and</strong> understood as wellas where to obtain further advice <strong>and</strong> support if needed. We are alsotalking to our customers <strong>and</strong> other stakeholders to explain thechanges they are seeing to the way we are working with them.We have comprehensive processes in place for monitoringcompliance with our Code of Conduct <strong>and</strong> global policies, includingdedicated compliance professionals who support our line managerslocally in monitoring their staff activities. We also have a nominatedsignatory network that works to ensure that our promotional materialsmeet all applicable requirements.Instances of potential non-compliance are collected through ourcompliance incident management processes <strong>and</strong> reviewed by seniormanagement in local <strong>and</strong>/or regional compliance committees. Seriousbreaches are reviewed by the Audit Committee <strong>and</strong>, if appropriate, theBoard. More information about our compliance <strong>and</strong> risk assuranceprocesses is contained in the Managing risk section from page 129.We take all breaches very seriously <strong>and</strong> act to prevent repeatoccurrences. In <strong>20</strong>11, we identified a total of 17 confirmed breaches ofexternal sales <strong>and</strong> marketing regulations or codes globally (<strong>20</strong>10: 11;<strong>20</strong>09: 24). Excluding the confirmed external breaches, there were1,275 instances of failure to comply with our Code of Conduct <strong>and</strong>global policies in our Commercial organisation, including contractstaff. In relation to all these breaches we removed 214 people fromtheir role, formally warned 570 people, <strong>and</strong> provided further guidanceor coaching on our policies for 971 people. It is important to note thata single breach can involve more than one employee failing to meetthe st<strong>and</strong>ards required.We believe that the increase in identified breaches is due in part toour enhanced management oversight of compliance <strong>and</strong> heightenedawareness of policy requirements through targeted training, alongsideimproved data capture mechanisms. However, we acknowledge thatour numbers are likely to continue to vary as we reshape our business<strong>and</strong> geographic footprint. We will continue to focus our complianceefforts appropriately.<strong>AstraZeneca</strong> <strong>Annual</strong> <strong>Report</strong> <strong>and</strong> <strong>Form</strong> <strong>20</strong>-F <strong>Information</strong> <strong>20</strong>11Disciplinary actions: Breaches of Code of Conduct by ourCommercial organisation including contract staffNumber of peopleAction taken <strong>20</strong>11 <strong>20</strong>10Removed from role* 214 117<strong>Form</strong>al warning 570 740Guidance <strong>and</strong> coaching 971 768Total 1,755 1,625* In the majority of cases, this means dismissal from the Company/contract termination, but it caninclude resignations <strong>and</strong> demotions.US Corporate Integrity Agreement reportingIn April <strong>20</strong>10, <strong>AstraZeneca</strong> signed an agreement with the USDepartment of Justice to settle an investigation relating to thesales <strong>and</strong> marketing of Seroquel IR. The requirements of theassociated Corporate Integrity Agreement between <strong>AstraZeneca</strong> <strong>and</strong>the Office of the Inspector General of the US Department of Health<strong>and</strong> Human Services (OIG) include a number of active monitoring<strong>and</strong> self-reporting obligations that differ from self-reporting requiredby authorities in the rest of the world. To meet these obligations,<strong>AstraZeneca</strong> provides notices to the OIG describing the outcomesof particular investigations potentially relating to violations of certainlaws, as well as a separate annual report to the OIG summarisingmonitoring <strong>and</strong> investigation outcomes relevant to Corporate IntegrityAgreement requirements.Access to healthcareProviding sustainable access to healthcare for all those who need itis a significant global challenge. The complexities surrounding theissue mean that there is no ‘one size fits all’ solution. Factors affectingaccess range from the affordability of medicines to the availabilityof healthcare systems <strong>and</strong> the resources to make them effective.We believe it will take a combined global effort involving all relatedstakeholders to drive sustainable progress in increasing access tohealthcare worldwide <strong>and</strong> we know that, as a global biopharmaceuticalcompany, we can make a meaningful contribution to that effort.Our strategy takes account of the different barriers to healthcare indifferent parts of the world <strong>and</strong>, because access to healthcare canalso vary within a country, our approach is tailored locally to meet theneeds of different patient populations. We are pursuing a range ofdifferent initiatives across these populations to underst<strong>and</strong> what worksbest <strong>and</strong> in what context. We believe we will be able to make thebiggest contribution to improving health where we are able to adopta commercial approach. Our goal is always to improve health forpatients <strong>and</strong> add value for our stakeholders <strong>and</strong> our business.> Our mainstream business will continue to focus on those peoplefor whom healthcare is readily available <strong>and</strong> who can afford ourmedicines. The selling of these medicines in our EstablishedMarkets helps enable us to generate the revenue we needto provide our shareholders with a return, invest in continuedinnovation <strong>and</strong> pursue other opportunities to increase theavailability of our medicines.Delivering our strategy Responsible Business 51Business Review