AstraZeneca Annual Report and Form 20-F Information 2011
AstraZeneca Annual Report and Form 20-F Information 2011
AstraZeneca Annual Report and Form 20-F Information 2011
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
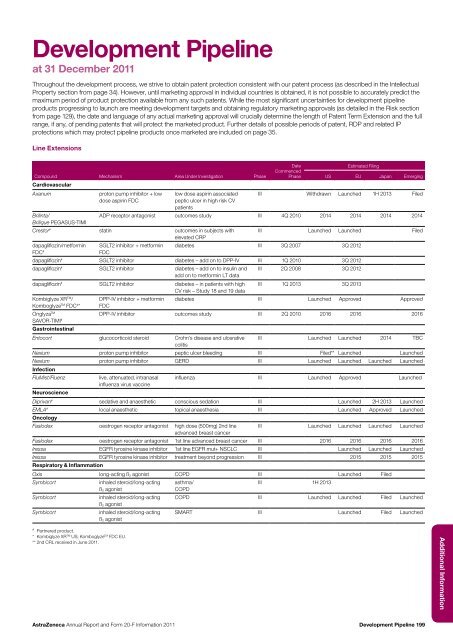
Development Pipelineat 31 December <strong>20</strong>11Throughout the development process, we strive to obtain patent protection consistent with our patent process (as described in the IntellectualProperty section from page 34). However, until marketing approval in individual countries is obtained, it is not possible to accurately predict themaximum period of product protection available from any such patents. While the most significant uncertainties for development pipelineproducts progressing to launch are meeting development targets <strong>and</strong> obtaining regulatory marketing approvals (as detailed in the Risk sectionfrom page 129), the date <strong>and</strong> language of any actual marketing approval will crucially determine the length of Patent Term Extension <strong>and</strong> the fullrange, if any, of pending patents that will protect the marketed product. Further details of possible periods of patent, RDP <strong>and</strong> related IPprotections which may protect pipeline products once marketed are included on page 35.Line ExtensionsDateEstimated FilingCompound Mechanism Area Under Investigation PhaseCommencedPhaseUS EU Japan EmergingCardiovascularAxanumproton pump inhibitor + low low dose aspirin associated III Withdrawn Launched 1H <strong>20</strong>13 Fileddose aspirin FDCpeptic ulcer in high risk CVpatientsBrilinta/ADP receptor antagonist outcomes study III 4Q <strong>20</strong>10 <strong>20</strong>14 <strong>20</strong>14 <strong>20</strong>14 <strong>20</strong>14Brilique PEGASUS-TIMICrestor # statin outcomes in subjects withelevated CRPIII Launched Launched Fileddapagliflozin/metforminFDC #SGLT2 inhibitor + metforminFDCdiabetes III 3Q <strong>20</strong>07 3Q <strong>20</strong>12dapagliflozin # SGLT2 inhibitor diabetes – add on to DPP-IV III 1Q <strong>20</strong>10 3Q <strong>20</strong>12dapagliflozin # SGLT2 inhibitor diabetes – add on to insulin <strong>and</strong> III 2Q <strong>20</strong>08 3Q <strong>20</strong>12add on to metformin LT datadapagliflozin # SGLT2 inhibitor diabetes – in patients with high III 1Q <strong>20</strong>13 3Q <strong>20</strong>13CV risk – Study 18 <strong>and</strong> 19 dataKombiglyze XR TM / DPP-IV inhibitor + metformin diabetes III Launched Approved ApprovedKomboglyze TM FDC # * FDCOnglyza TMDPP-IV inhibitor outcomes study III 2Q <strong>20</strong>10 <strong>20</strong>16 <strong>20</strong>16 <strong>20</strong>16SAVOR-TIMI #GastrointestinalEntocort glucocorticoid steroid Crohn’s disease <strong>and</strong> ulcerative III Launched Launched <strong>20</strong>14 TBCcolitisNexium proton pump inhibitor peptic ulcer bleeding III Filed** Launched LaunchedNexium proton pump inhibitor GERD III Launched Launched Launched LaunchedInfectionFluMist/Fluenzlive, attenuated, intranasal influenza III Launched Approved Launchedinfluenza virus vaccineNeuroscienceDiprivan # sedative <strong>and</strong> anaesthetic conscious sedation III Launched 2H <strong>20</strong>13 LaunchedEMLA # local anaesthetic topical anaesthesia III Launched Approved LaunchedOncologyFaslodex oestrogen receptor antagonist high dose (500mg) 2nd line III Launched Launched Launched Launchedadvanced breast cancerFaslodex oestrogen receptor antagonist 1st line advanced breast cancer III <strong>20</strong>16 <strong>20</strong>16 <strong>20</strong>16 <strong>20</strong>16Iressa EGFR tyrosine kinase inhibitor 1st line EGFR mut+ NSCLC III Launched Launched LaunchedIressa EGFR tyrosine kinase inhibitor treatment beyond progression III <strong>20</strong>15 <strong>20</strong>15 <strong>20</strong>15Respiratory & InflammationOxis long-acting ß 2 agonist COPD III Launched FiledSymbicortSymbicortSymbicortinhaled steroid/long-actingß 2 agonistinhaled steroid/long-actingß 2 agonistinhaled steroid/long-actingß 2 agonistasthma/III 1H <strong>20</strong>13COPDCOPD III Launched Launched Filed LaunchedSMART III Launched Filed Launched# Partnered product.* Kombiglyze XR TM US; Komboglyze TM FDC EU.** 2nd CRL received in June <strong>20</strong>11.Additional <strong>Information</strong><strong>AstraZeneca</strong> <strong>Annual</strong> <strong>Report</strong> <strong>and</strong> <strong>Form</strong> <strong>20</strong>-F <strong>Information</strong> <strong>20</strong>11Development Pipeline 199