AstraZeneca Annual Report and Form 20-F Information 2011
AstraZeneca Annual Report and Form 20-F Information 2011
AstraZeneca Annual Report and Form 20-F Information 2011
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
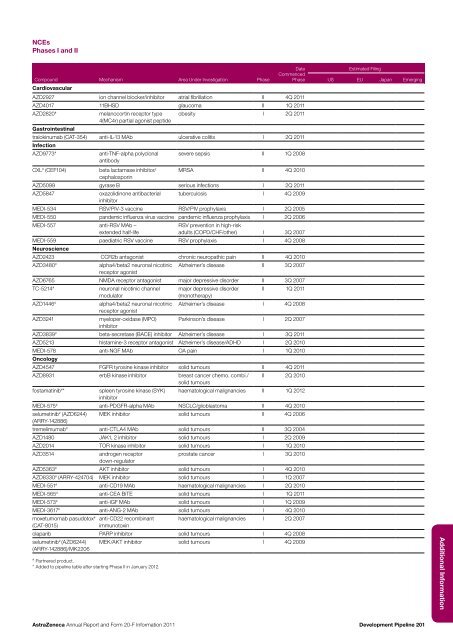
NCEsPhases I <strong>and</strong> IICompound Mechanism Area Under Investigation PhaseDateCommencedPhaseCardiovascularAZD2927 ion channel blocker/inhibitor atrial fibrillation II 4Q <strong>20</strong>11AZD4017 11BHSD glaucoma II 1Q <strong>20</strong>11AZD28<strong>20</strong> #melanocortin receptor type obesity I 2Q <strong>20</strong>114(MC4r) partial agonist peptideGastrointestinaltralokinumab (CAT-354) anti-IL-13 MAb ulcerative colitis I 2Q <strong>20</strong>11InfectionAZD9773 #anti-TNF-alpha polyclonal severe sepsis II 1Q <strong>20</strong>08antibodyCXL # (CEF104)beta lactamase inhibitor/ MRSA II 4Q <strong>20</strong>10cephalosporinAZD5099 gyrase B serious infections I 2Q <strong>20</strong>11AZD5847oxazolidinone antibacterial tuberculosis I 4Q <strong>20</strong>09inhibitorMEDI-534 RSV/PIV-3 vaccine RSV/PIV prophylaxis I 2Q <strong>20</strong>05MEDI-550 p<strong>and</strong>emic influenza virus vaccine p<strong>and</strong>emic influenza prophylaxis I 2Q <strong>20</strong>06MEDI-557 anti-RSV MAb –extended half-lifeRSV prevention in high-riskadults (COPD/CHF/other) I 3Q <strong>20</strong>07MEDI-559 paediatric RSV vaccine RSV prophylaxis I 4Q <strong>20</strong>08NeuroscienceAZD2423 CCR2b antagonist chronic neuropathic pain II 4Q <strong>20</strong>10AZD3480 #alpha4/beta2 neuronal nicotinic Alzheimer’s disease II 3Q <strong>20</strong>07receptor agonistAZD6765 NMDA receptor antagonist major depressive disorder II 3Q <strong>20</strong>07TC-5214 #neuronal nicotinic channel major depressive disorder II 1Q <strong>20</strong>11modulator(monotherapy)AZD1446 #alpha4/beta2 neuronal nicotinic Alzheimer’s disease I 4Q <strong>20</strong>08receptor agonistAZD3241myeloper-oxidase (MPO) Parkinson’s disease I 2Q <strong>20</strong>07inhibitorAZD3839 # beta-secretase (BACE) inhibitor Alzheimer’s disease I 3Q <strong>20</strong>11AZD5213 histamine-3 receptor antagonist Alzheimer’s disease/ADHD I 2Q <strong>20</strong>10MEDI-578 anti-NGF MAb OA pain I 1Q <strong>20</strong>10OncologyAZD4547 FGFR tyrosine kinase inhibitor solid tumours II 4Q <strong>20</strong>11AZD8931 erbB kinase inhibitor breast cancer chemo. combi./ II 2Q <strong>20</strong>10solid tumoursfostamatinib # *spleen tyrosine kinase (SYK) haematological malignancies II 1Q <strong>20</strong>12inhibitorMEDI-575 # anti-PDGFR-alpha MAb NSCLC/glioblastoma II 4Q <strong>20</strong>10selumetinib # (AZD6244) MEK inhibitor solid tumours II 4Q <strong>20</strong>06(ARRY-142886)tremelimumab # anti-CTLA4 MAb solid tumours II 3Q <strong>20</strong>04AZD1480 JAK1, 2 inhibitor solid tumours I 2Q <strong>20</strong>09AZD<strong>20</strong>14 TOR kinase inhibitor solid tumours I 1Q <strong>20</strong>10AZD3514<strong>and</strong>rogen receptorprostate cancer I 3Q <strong>20</strong>10down-regulatorAZD5363 # AKT inhibitor solid tumours I 4Q <strong>20</strong>10AZD8330 # (ARRY-424704) MEK inhibitor solid tumours I 1Q <strong>20</strong>07MEDI-551 # anti-CD19 MAb haematological malignancies I 2Q <strong>20</strong>10MEDI-565 # anti-CEA BiTE solid tumours I 1Q <strong>20</strong>11MEDI-573 # anti-IGF MAb solid tumours I 1Q <strong>20</strong>09MEDI-3617 # anti-ANG-2 MAb solid tumours I 4Q <strong>20</strong>10moxetumomab pasudotox #(CAT-8015)anti-CD22 recombinantimmunotoxinhaematological malignancies I 2Q <strong>20</strong>07olaparib PARP inhibitor solid tumours I 4Q <strong>20</strong>08selumetinib # (AZD6244) MEK/AKT inhibitor solid tumours I 4Q <strong>20</strong>09(ARRY-142886)/MK2<strong>20</strong>6# Partnered product.* Added to pipeline table after starting Phase II in January <strong>20</strong>12.Estimated FilingUS EU Japan EmergingAdditional <strong>Information</strong><strong>AstraZeneca</strong> <strong>Annual</strong> <strong>Report</strong> <strong>and</strong> <strong>Form</strong> <strong>20</strong>-F <strong>Information</strong> <strong>20</strong>11Development Pipeline <strong>20</strong>1