AstraZeneca Annual Report and Form 20-F Information 2011
AstraZeneca Annual Report and Form 20-F Information 2011
AstraZeneca Annual Report and Form 20-F Information 2011
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
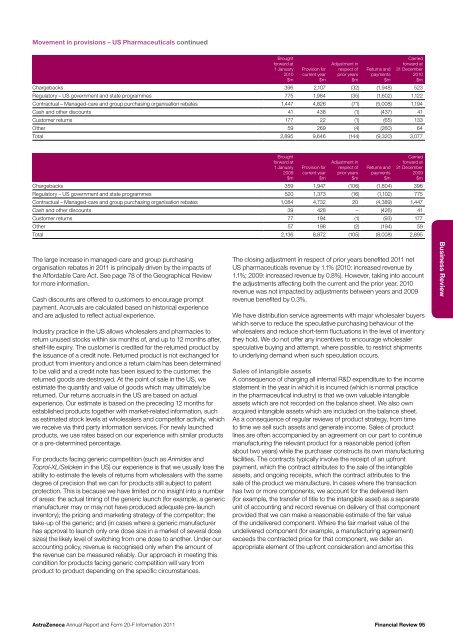
Movement in provisions – US Pharmaceuticals continuedBroughtforward at1 January<strong>20</strong>10$mProvision forcurrent year$mAdjustment inrespect ofprior years$mReturns <strong>and</strong>payments$mCarriedforward at31 December<strong>20</strong>10$mChargebacks 396 2,107 (32) (1,948) 523Regulatory – US government <strong>and</strong> state programmes 775 1,984 (35) (1,602) 1,122Contractual – Managed-care <strong>and</strong> group purchasing organisation rebates 1,447 4,826 (71) (5,008) 1,194Cash <strong>and</strong> other discounts 41 438 (1) (437) 41Customer returns 177 22 (1) (65) 133Other 59 269 (4) (260) 64Total 2,895 9,646 (144) (9,3<strong>20</strong>) 3,077Broughtforward at1 January<strong>20</strong>09$mProvision forcurrent year$mAdjustment inrespect ofprior years$mReturns <strong>and</strong>payments$mCarriedforward at31 December<strong>20</strong>09$mChargebacks 359 1,947 (106) (1,804) 396Regulatory – US government <strong>and</strong> state programmes 5<strong>20</strong> 1,373 (16) (1,102) 775Contractual – Managed-care <strong>and</strong> group purchasing organisation rebates 1,084 4,732 <strong>20</strong> (4,389) 1,447Cash <strong>and</strong> other discounts 39 428 – (426) 41Customer returns 77 194 (1) (93) 177Other 57 198 (2) (194) 59Total 2,136 8,872 (105) (8,008) 2,895The large increase in managed-care <strong>and</strong> group purchasingorganisation rebates in <strong>20</strong>11 is principally driven by the impacts ofthe Affordable Care Act. See page 78 of the Geographical Reviewfor more information.Cash discounts are offered to customers to encourage promptpayment. Accruals are calculated based on historical experience<strong>and</strong> are adjusted to reflect actual experience.Industry practice in the US allows wholesalers <strong>and</strong> pharmacies toreturn unused stocks within six months of, <strong>and</strong> up to 12 months after,shelf-life expiry. The customer is credited for the returned product bythe issuance of a credit note. Returned product is not exchanged forproduct from inventory <strong>and</strong> once a return claim has been determinedto be valid <strong>and</strong> a credit note has been issued to the customer, thereturned goods are destroyed. At the point of sale in the US, weestimate the quantity <strong>and</strong> value of goods which may ultimately bereturned. Our returns accruals in the US are based on actualexperience. Our estimate is based on the preceding 12 months forestablished products together with market-related information, suchas estimated stock levels at wholesalers <strong>and</strong> competitor activity, whichwe receive via third party information services. For newly launchedproducts, we use rates based on our experience with similar productsor a pre-determined percentage.For products facing generic competition (such as Arimidex <strong>and</strong>Toprol-XL/Seloken in the US) our experience is that we usually lose theability to estimate the levels of returns from wholesalers with the samedegree of precision that we can for products still subject to patentprotection. This is because we have limited or no insight into a numberof areas: the actual timing of the generic launch (for example, a genericmanufacturer may or may not have produced adequate pre-launchinventory); the pricing <strong>and</strong> marketing strategy of the competitor; thetake-up of the generic; <strong>and</strong> (in cases where a generic manufacturerhas approval to launch only one dose size in a market of several dosesizes) the likely level of switching from one dose to another. Under ouraccounting policy, revenue is recognised only when the amount ofthe revenue can be measured reliably. Our approach in meeting thiscondition for products facing generic competition will vary fromproduct to product depending on the specific circumstances.The closing adjustment in respect of prior years benefited <strong>20</strong>11 netUS pharmaceuticals revenue by 1.1% (<strong>20</strong>10: increased revenue by1.1%; <strong>20</strong>09: increased revenue by 0.8%). However, taking into accountthe adjustments affecting both the current <strong>and</strong> the prior year, <strong>20</strong>10revenue was not impacted by adjustments between years <strong>and</strong> <strong>20</strong>09revenue benefited by 0.3%.We have distribution service agreements with major wholesaler buyerswhich serve to reduce the speculative purchasing behaviour of thewholesalers <strong>and</strong> reduce short-term fluctuations in the level of inventorythey hold. We do not offer any incentives to encourage wholesalerspeculative buying <strong>and</strong> attempt, where possible, to restrict shipmentsto underlying dem<strong>and</strong> when such speculation occurs.Sales of intangible assetsA consequence of charging all internal R&D expenditure to the incomestatement in the year in which it is incurred (which is normal practicein the pharmaceutical industry) is that we own valuable intangibleassets which are not recorded on the balance sheet. We also ownacquired intangible assets which are included on the balance sheet.As a consequence of regular reviews of product strategy, from timeto time we sell such assets <strong>and</strong> generate income. Sales of productlines are often accompanied by an agreement on our part to continuemanufacturing the relevant product for a reasonable period (oftenabout two years) while the purchaser constructs its own manufacturingfacilities. The contracts typically involve the receipt of an upfrontpayment, which the contract attributes to the sale of the intangibleassets, <strong>and</strong> ongoing receipts, which the contract attributes to thesale of the product we manufacture. In cases where the transactionhas two or more components, we account for the delivered item(for example, the transfer of title to the intangible asset) as a separateunit of accounting <strong>and</strong> record revenue on delivery of that componentprovided that we can make a reasonable estimate of the fair valueof the undelivered component. Where the fair market value of theundelivered component (for example, a manufacturing agreement)exceeds the contracted price for that component, we defer anappropriate element of the upfront consideration <strong>and</strong> amortise thisBusiness Review<strong>AstraZeneca</strong> <strong>Annual</strong> <strong>Report</strong> <strong>and</strong> <strong>Form</strong> <strong>20</strong>-F <strong>Information</strong> <strong>20</strong>11Financial Review 95