Research Group Heussler (Malaria I) - Bernhard-Nocht-Institut für ...
Research Group Heussler (Malaria I) - Bernhard-Nocht-Institut für ...
Research Group Heussler (Malaria I) - Bernhard-Nocht-Institut für ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Parasitology Section<br />
Molecular Basis of Miltefosine Resistance<br />
in Leishmaniasis<br />
Zusammenfassung<br />
Wir streben die Identifizierung der genetischen<br />
Grundlagen <strong>für</strong> die Resistenz von Leishmania-Parasiten<br />
gegen den neuen Antileishmania-Wirkstoff Miltefosine<br />
an. Zu diesem Zweck haben wir ein screening<br />
durch funktionelle Komplementation suszeptibler<br />
Parasiten durchgeführt. Bis jetzt konnten wenigstens<br />
zwei unabhängige Genloci identifiziert werden, deren<br />
Amplifikation zur Miltefosin-Reisistenz in vitro<br />
führt. Ziel ist die Entwicklung proaktiver Strategien<br />
gegen die Entwicklung von klinischer Therapieresistenz.<br />
Summary<br />
We aim at the identification of genetic traits that lead to<br />
resistance to the new antileishmanial drug miltefosine.<br />
To this end, we have implemented a screen using functional<br />
complementation genetics. So far, at least two independent<br />
gene loci were identified that can, upon<br />
overexpression, confer miltefosine resistance in vitro.<br />
The goal is the development of a proactive strategy<br />
against the development of clinical resistance to this<br />
newly approved drug.<br />
38<br />
Introduction<br />
Visceral leishmaniasis (VL) or Kala Azar (KA) is caused<br />
by Leishmania donovani and L. infantum and is reported<br />
from about 47 countries around the world. It has<br />
an annual incidence of 500,000, and 90% of the cases<br />
occur in just four countries: India, Nepal, Bangladesh<br />
and Sudan. In India, the neighbour states of Bihar, Uttar<br />
Pradesh and West Bengal are highly endemic foci of<br />
KA where periodic epidemics are common due to anthroponotic<br />
transmission cycles. Over 60% of the cases<br />
in Northern Bihar are resistant to traditional antimony<br />
therapies and respond only to the expensive Amphotericin<br />
B treatment. In India and in other endemic regions,<br />
the number of cases of Leishmania-HIV co-infections<br />
is increasing due to the simultaneous spread and<br />
geographic overlap of both diseases. This new clinical<br />
entity is refractory to chemotherapy. This and the increased<br />
transmission of Leishmania spp. through contaminated<br />
syringes may further contribute to the spread<br />
of therapy resistance.<br />
A new, orally administered drug, miltefosine, has recently<br />
been approved for treatment of KA in India. Miltefosine<br />
had been developed as an anti-breast cancer<br />
drug, but it shows good efficacy against Leishmania<br />
donovani both in vitro and in vivo. However, first reports<br />
have emerged of miltefosine resistant breast cancer<br />
cell lines and a resistant Leishmania strain. Miltefosine<br />
appears to be stable in the human organism at subtherapeutic<br />
doses for prolonged periods after treatment,<br />
raising concerns about the possible development<br />
of resistance in anthroponotic VL.<br />
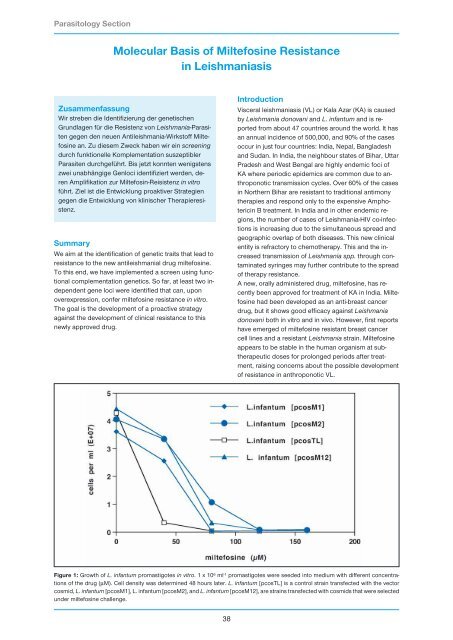
Figure 1: Growth of L. infantum promastigotes in vitro. 1 x 10 6 ml -1 promastigotes were seeded into medium with different concentrations<br />
of the drug (µM). Cell density was determined 48 hours later. L. infantum [pcosTL] is a control strain transfected with the vector<br />
cosmid, L. infantum [pcosM1], L. infantum [pcosM2], and L. infantum [pcosM12], are strains transfected with cosmids that were selected<br />
under miltefosine challenge.