- Page 1:
PHARMACEUTICAL MANUFACTURING HANDBO
- Page 5 and 6:
PHARMACEUTICAL MANUFACTURING HANDBO
- Page 7 and 8:
CONTRIBUTORS Susanna Abrahms é n -
- Page 9 and 10:
CONTRIBUTORS vii Eddy Castellanos G
- Page 11 and 12:
CONTENTS PREFACE xiii SECTION 1 MAN
- Page 13:
CONTENTS xi 5.11 Transdermal Drug D
- Page 17:
SECTION 1 MANUFACTURING SPECIALTIES
- Page 20 and 21:
4 BIOTECHNOLOGY-DERIVED DRUG PRODUC
- Page 22 and 23:
6 BIOTECHNOLOGY-DERIVED DRUG PRODUC
- Page 24 and 25:
8 BIOTECHNOLOGY-DERIVED DRUG PRODUC
- Page 26 and 27:
10 BIOTECHNOLOGY-DERIVED DRUG PRODU
- Page 28 and 29:
12 BIOTECHNOLOGY-DERIVED DRUG PRODU
- Page 30 and 31:
14 BIOTECHNOLOGY-DERIVED DRUG PRODU
- Page 32 and 33:
16 BIOTECHNOLOGY-DERIVED DRUG PRODU
- Page 34 and 35:
18 BIOTECHNOLOGY-DERIVED DRUG PRODU
- Page 36 and 37:
20 BIOTECHNOLOGY-DERIVED DRUG PRODU
- Page 38 and 39:
22 BIOTECHNOLOGY-DERIVED DRUG PRODU
- Page 40 and 41:
24 BIOTECHNOLOGY-DERIVED DRUG PRODU
- Page 42 and 43:
26 BIOTECHNOLOGY-DERIVED DRUG PRODU
- Page 44 and 45:
28 BIOTECHNOLOGY-DERIVED DRUG PRODU
- Page 46 and 47:
30 BIOTECHNOLOGY-DERIVED DRUG PRODU
- Page 48 and 49:
32 BIOTECHNOLOGY-DERIVED DRUG PRODU
- Page 50 and 51:
34 REGULATORY CONSIDERATIONS IN APP
- Page 52 and 53:
36 REGULATORY CONSIDERATIONS IN APP
- Page 54 and 55:
38 REGULATORY CONSIDERATIONS IN APP
- Page 56 and 57:
40 REGULATORY CONSIDERATIONS IN APP
- Page 58 and 59:
42 REGULATORY CONSIDERATIONS IN APP
- Page 60 and 61:
44 REGULATORY CONSIDERATIONS IN APP
- Page 62 and 63:
46 REGULATORY CONSIDERATIONS IN APP
- Page 64 and 65:
48 REGULATORY CONSIDERATIONS IN APP
- Page 66 and 67:
50 REGULATORY CONSIDERATIONS IN APP
- Page 68 and 69:
52 REGULATORY CONSIDERATIONS IN APP
- Page 70 and 71:
54 REGULATORY CONSIDERATIONS IN APP
- Page 72 and 73:
56 REGULATORY CONSIDERATIONS IN APP
- Page 75 and 76:
1.3 RADIOPHARMACEUTICAL MANUFACTURI
- Page 77 and 78:
The terms tracer, radiotracer , and
- Page 79 and 80:
number of atoms that disintegrate d
- Page 81 and 82:
images that can also give quantitat
- Page 83 and 84:
PRODUCT DEVELOPMENT 67 Product Stab
- Page 85 and 86:
MANUFACTURING ASPECTS 69 stations s
- Page 87 and 88:
In general, the manufacturing of mo
- Page 89 and 90:
MANUFACTURING ASPECTS 73 Production
- Page 91 and 92:
PRODUCT MANUFACTURING 75 tainer. Th
- Page 93 and 94:
PRODUCT MANUFACTURING 77 practical
- Page 95 and 96:
PRODUCT MANUFACTURING 79 these radi
- Page 97 and 98:
PRODUCT MANUFACTURING 81 Most of th
- Page 99 and 100:
PRODUCT MANUFACTURING 83 PET Radiop
- Page 101 and 102:
PRODUCT MANUFACTURING 85 FIGURE 5 S
- Page 103 and 104:
PRODUCT MANUFACTURING 87 tions in t
- Page 105 and 106:
QUALITY CONSIDERATIONS 89 regulatio
- Page 107 and 108:
L1 L2 TC-MDP + Hydr. Tc TC-MDP + Tc
- Page 109 and 110:
EXTEMPORANEOUS PREPARATION OF RADIO
- Page 111 and 112:
Independent of which regulation app
- Page 113:
SECTION 2 ASEPTIC PROCESSING
- Page 116 and 117:
100 STERILE PRODUCT MANUFACTURING 2
- Page 118 and 119:
102 STERILE PRODUCT MANUFACTURING A
- Page 120 and 121:
104 STERILE PRODUCT MANUFACTURING T
- Page 122 and 123:
106 STERILE PRODUCT MANUFACTURING E
- Page 124 and 125:
108 STERILE PRODUCT MANUFACTURING I
- Page 126 and 127:
110 STERILE PRODUCT MANUFACTURING 2
- Page 128 and 129:
112 STERILE PRODUCT MANUFACTURING c
- Page 130 and 131:
114 STERILE PRODUCT MANUFACTURING 2
- Page 132 and 133:
116 STERILE PRODUCT MANUFACTURING 2
- Page 134 and 135:
118 STERILE PRODUCT MANUFACTURING i
- Page 136 and 137:
120 STERILE PRODUCT MANUFACTURING o
- Page 138 and 139:
122 STERILE PRODUCT MANUFACTURING a
- Page 140 and 141:
124 STERILE PRODUCT MANUFACTURING a
- Page 142 and 143:
126 STERILE PRODUCT MANUFACTURING e
- Page 144 and 145:
128 STERILE PRODUCT MANUFACTURING A
- Page 146 and 147:
130 STERILE PRODUCT MANUFACTURING 2
- Page 148 and 149:
132 STERILE PRODUCT MANUFACTURING p
- Page 150 and 151:
134 STERILE PRODUCT MANUFACTURING 2
- Page 153:
SECTION 3 FACILITY
- Page 156 and 157:
140 EFFECT OF SCALE-UP ON OPERATION
- Page 158 and 159:
142 EFFECT OF SCALE-UP ON OPERATION
- Page 160 and 161:
144 EFFECT OF SCALE-UP ON OPERATION
- Page 162 and 163:
146 EFFECT OF SCALE-UP ON OPERATION
- Page 164 and 165:
148 EFFECT OF SCALE-UP ON OPERATION
- Page 166 and 167:
150 EFFECT OF SCALE-UP ON OPERATION
- Page 168 and 169:
152 EFFECT OF SCALE-UP ON OPERATION
- Page 170 and 171:
154 EFFECT OF SCALE-UP ON OPERATION
- Page 172 and 173:
156 EFFECT OF SCALE-UP ON OPERATION
- Page 174 and 175:
158 EFFECT OF SCALE-UP ON OPERATION
- Page 176 and 177:
160 PACKAGING AND LABELING 3.2.1 3.
- Page 178 and 179:
162 PACKAGING AND LABELING signifi
- Page 180 and 181:
164 PACKAGING AND LABELING Although
- Page 182 and 183:
166 PACKAGING AND LABELING componen
- Page 184 and 185:
168 PACKAGING AND LABELING sions. T
- Page 186 and 187:
170 PACKAGING AND LABELING TABLE 1
- Page 188 and 189:
172 PACKAGING AND LABELING the stab
- Page 190 and 191:
174 PACKAGING AND LABELING and accu
- Page 192 and 193:
176 PACKAGING AND LABELING The safe
- Page 194 and 195:
178 PACKAGING AND LABELING If the p
- Page 196 and 197:
180 PACKAGING AND LABELING exposure
- Page 198 and 199:
182 PACKAGING AND LABELING In many
- Page 200 and 201:
184 PACKAGING AND LABELING Nonpharm
- Page 202 and 203:
186 PACKAGING AND LABELING In 1906,
- Page 204 and 205:
188 PACKAGING AND LABELING A medica
- Page 206 and 207:
190 PACKAGING AND LABELING The inst
- Page 208 and 209:
192 PACKAGING AND LABELING the poun
- Page 210 and 211:
194 PACKAGING AND LABELING • (4)
- Page 212 and 213:
196 PACKAGING AND LABELING In the c
- Page 214 and 215:
198 PACKAGING AND LABELING • Inte
- Page 216 and 217:
200 PACKAGING AND LABELING 27. U.S.
- Page 218 and 219:
202 CLEAN-FACILITY DESIGN, CONSTRUC
- Page 220 and 221:
204 CLEAN-FACILITY DESIGN, CONSTRUC
- Page 222 and 223:
206 CLEAN-FACILITY DESIGN, CONSTRUC
- Page 224 and 225:
208 CLEAN-FACILITY DESIGN, CONSTRUC
- Page 226 and 227:
210 CLEAN-FACILITY DESIGN, CONSTRUC
- Page 228 and 229:
212 CLEAN-FACILITY DESIGN, CONSTRUC
- Page 230 and 231:
214 CLEAN-FACILITY DESIGN, CONSTRUC
- Page 232 and 233:
216 CLEAN-FACILITY DESIGN, CONSTRUC
- Page 234 and 235:
218 CLEAN-FACILITY DESIGN, CONSTRUC
- Page 236 and 237:
220 CLEAN-FACILITY DESIGN, CONSTRUC
- Page 238 and 239: 222 CLEAN-FACILITY DESIGN, CONSTRUC
- Page 240 and 241: 224 CLEAN-FACILITY DESIGN, CONSTRUC
- Page 242 and 243: 226 CLEAN-FACILITY DESIGN, CONSTRUC
- Page 244 and 245: 228 CLEAN-FACILITY DESIGN, CONSTRUC
- Page 246 and 247: 230 CLEAN-FACILITY DESIGN, CONSTRUC
- Page 248 and 249: 232 CLEAN-FACILITY DESIGN, CONSTRUC
- Page 251 and 252: 4.1 SOLID DOSAGE FORMS Barbara R. C
- Page 253 and 254: TABLE 2 Types of Solid Dosage Form
- Page 255 and 256: (GI) tract or for systemic effects.
- Page 257 and 258: EXCIPIENTS IN SOLID DOSE FORMULATIO
- Page 259 and 260: EXCIPIENTS IN SOLID DOSE FORMULATIO
- Page 261 and 262: HARD AND SOFT GELATIN CAPSULES 245
- Page 263 and 264: HARD AND SOFT GELATIN CAPSULES 247
- Page 265 and 266: HARD AND SOFT GELATIN CAPSULES 249
- Page 267 and 268: participants from the FDA, industry
- Page 269 and 270: in the mouth. Compressed lozenges (
- Page 271 and 272: ittle fi lm, have no unpleasant tas
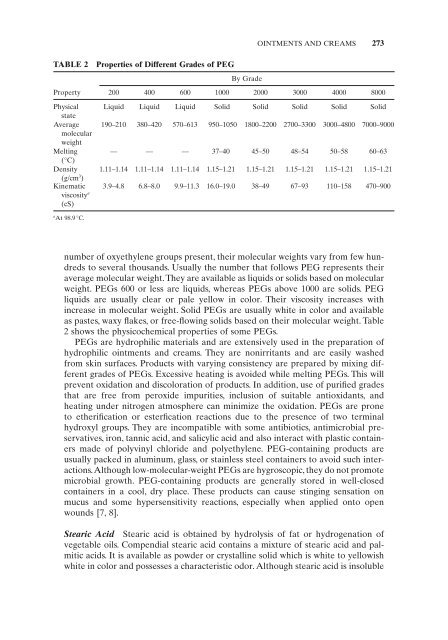
- Page 273 and 274: CHEWING GUMS 257 4.1.10.2 Manufactu
- Page 275 and 276: already dissolved in the saliva pri
- Page 277 and 278: ORALLY DISINTEGRATING TABLETS 261 a
- Page 279 and 280: tions. Some buccal formulations hav
- Page 281: REFERENCES 265 32. Habib , W. , Kha
- Page 284 and 285: 268 SEMISOLID DOSAGES: OINTMENTS, C
- Page 286 and 287: 270 SEMISOLID DOSAGES: OINTMENTS, C
- Page 290 and 291: 274 SEMISOLID DOSAGES: OINTMENTS, C
- Page 292 and 293: 276 SEMISOLID DOSAGES: OINTMENTS, C
- Page 294 and 295: 278 SEMISOLID DOSAGES: OINTMENTS, C
- Page 296 and 297: 280 SEMISOLID DOSAGES: OINTMENTS, C
- Page 298 and 299: 282 SEMISOLID DOSAGES: OINTMENTS, C
- Page 300 and 301: 284 SEMISOLID DOSAGES: OINTMENTS, C
- Page 302 and 303: 286 SEMISOLID DOSAGES: OINTMENTS, C
- Page 304 and 305: 288 SEMISOLID DOSAGES: OINTMENTS, C
- Page 306 and 307: 290 SEMISOLID DOSAGES: OINTMENTS, C
- Page 308 and 309: 292 SEMISOLID DOSAGES: OINTMENTS, C
- Page 310 and 311: 294 SEMISOLID DOSAGES: OINTMENTS, C
- Page 312 and 313: 296 SEMISOLID DOSAGES: OINTMENTS, C
- Page 314 and 315: 298 SEMISOLID DOSAGES: OINTMENTS, C
- Page 316 and 317: 300 SEMISOLID DOSAGES: OINTMENTS, C
- Page 318 and 319: 302 SEMISOLID DOSAGES: OINTMENTS, C
- Page 320 and 321: 304 SEMISOLID DOSAGES: OINTMENTS, C
- Page 322 and 323: 306 SEMISOLID DOSAGES: OINTMENTS, C
- Page 324 and 325: 308 SEMISOLID DOSAGES: OINTMENTS, C
- Page 326 and 327: 310 SEMISOLID DOSAGES: OINTMENTS, C
- Page 328 and 329: 312 SEMISOLID DOSAGES: OINTMENTS, C
- Page 330 and 331: 314 LIQUID DOSAGE FORMS 4.3.1 INTRO
- Page 332 and 333: 316 LIQUID DOSAGE FORMS 4.3.2 GENER
- Page 334 and 335: 318 LIQUID DOSAGE FORMS design, bui
- Page 336 and 337: 320 LIQUID DOSAGE FORMS tions with
- Page 338 and 339:
322 LIQUID DOSAGE FORMS Dosing Pump
- Page 340 and 341:
324 LIQUID DOSAGE FORMS However, th
- Page 342 and 343:
326 LIQUID DOSAGE FORMS Location of
- Page 344 and 345:
328 LIQUID DOSAGE FORMS Solid drugs
- Page 346 and 347:
330 LIQUID DOSAGE FORMS TABLE 3 Emu
- Page 348 and 349:
332 LIQUID DOSAGE FORMS Temperature
- Page 350 and 351:
334 LIQUID DOSAGE FORMS for viscosi
- Page 352 and 353:
336 LIQUID DOSAGE FORMS spectrum, s
- Page 354 and 355:
338 LIQUID DOSAGE FORMS Product Spe
- Page 356 and 357:
340 LIQUID DOSAGE FORMS Liquid, Ext
- Page 358 and 359:
342 LIQUID DOSAGE FORMS 7. Kourouna
- Page 360 and 361:
344 LIQUID DOSAGE FORMS 46. Miller
- Page 363 and 364:
5.1 CONTROLLED - RELEASE DOSAGE FOR
- Page 365 and 366:
CONTROLLED-RELEASE DRUG DELIVERY SY
- Page 367 and 368:
CONTROLLED-RELEASE FORMULATIONS 351
- Page 369 and 370:
dosage forms [12] . For example, or
- Page 371 and 372:
Degradation Liver Hydrolytic Enzyma
- Page 373 and 374:
CONTROLLED-RELEASE ORAL DOSAGE FORM
- Page 375 and 376:
DESIGN AND FABRICATION OF CONTROLLE
- Page 377 and 378:
DESIGN AND FABRICATION OF CONTROLLE
- Page 379 and 380:
DESIGN AND FABRICATION OF CONTROLLE
- Page 381 and 382:
DESIGN AND FABRICATION OF CONTROLLE
- Page 383 and 384:
TECHNOLOGIES FOR DEVELOPING TRANSDE
- Page 385 and 386:
TECHNOLOGIES FOR DEVELOPING TRANSDE
- Page 387 and 388:
RELEASE OF DRUGS FROM CONTROLLED-RE
- Page 389 and 390:
RELEASE OF DRUGS FROM CONTROLLED-RE
- Page 391 and 392:
RELEASE OF DRUGS FROM CONTROLLED-RE
- Page 393 and 394:
RELEASE OF DRUGS FROM CONTROLLED-RE
- Page 395 and 396:
RELEASE OF DRUGS FROM CONTROLLED-RE
- Page 397 and 398:
RELEASE OF DRUGS FROM CONTROLLED-RE
- Page 399 and 400:
RELEASE OF DRUGS FROM CONTROLLED-RE
- Page 401 and 402:
RELEASE OF DRUGS FROM CONTROLLED-RE
- Page 403 and 404:
REFERENCES 387 16. Anal , A. K. ( 2
- Page 405 and 406:
REFERENCES 389 57. M ü ller , R. H
- Page 407 and 408:
REFERENCES 391 96. Sungthongjeen ,
- Page 409 and 410:
5.2 PROGRESS IN DESIGN OF BIODEGRAD
- Page 411 and 412:
INTRODUCTION 395 sequences. In addi
- Page 413 and 414:
TABLE 2 Injectable Peptide/Proteins
- Page 415 and 416:
PEPTIDE/PROTEIN-LOADED MICROSPHERE
- Page 417 and 418:
PEPTIDE/PROTEIN-LOADED MICROSPHERE
- Page 419 and 420:
PEPTIDE/PROTEIN-LOADED MICROSPHERE
- Page 421 and 422:
ANALYTICAL CHARACTERIZATION 405 imm
- Page 423 and 424:
IMMUNE SYSTEM INTERACTION WITH INJE
- Page 425 and 426:
INJECTABLE PEPTIDE/PROTEIN-LOADED M
- Page 427 and 428:
INJECTABLE PEPTIDE/PROTEIN-LOADED M
- Page 429 and 430:
INJECTABLE PEPTIDE/PROTEIN-LOADED M
- Page 431 and 432:
INJECTABLE PEPTIDE/PROTEIN-LOADED M
- Page 433 and 434:
INJECTABLE PEPTIDE/PROTEIN-LOADED M
- Page 435 and 436:
PEPTIDE/PROTEIN ENCAPSULATED INTO B
- Page 437 and 438:
PEPTIDE/PROTEIN ENCAPSULATED INTO B
- Page 439 and 440:
PEPTIDE/PROTEIN ENCAPSULATED INTO B
- Page 441 and 442:
PEPTIDE/PROTEIN ENCAPSULATED INTO B
- Page 443 and 444:
REFERENCES 427 novel ways to reduce
- Page 445 and 446:
REFERENCES 429 31. Morlock , M. , K
- Page 447 and 448:
REFERENCES 431 63. Lam , X. M. , Du
- Page 449 and 450:
REFERENCES 433 95. van de Weert , M
- Page 451 and 452:
REFERENCES 435 130. Bilati , U. , A
- Page 453 and 454:
REFERENCES 437 166. Means , G. E. ,
- Page 455 and 456:
REFERENCES 439 bic poly(lactide -co
- Page 457:
REFERENCES 441 237. Singh , M. , Li
- Page 460 and 461:
444 LIPOSOMES AND DRUG DELIVERY (sm
- Page 462 and 463:
446 LIPOSOMES AND DRUG DELIVERY Con
- Page 464 and 465:
448 LIPOSOMES AND DRUG DELIVERY and
- Page 466 and 467:
450 LIPOSOMES AND DRUG DELIVERY tec
- Page 468 and 469:
452 LIPOSOMES AND DRUG DELIVERY 250
- Page 470 and 471:
454 LIPOSOMES AND DRUG DELIVERY a s
- Page 472 and 473:
456 LIPOSOMES AND DRUG DELIVERY For
- Page 474 and 475:
458 LIPOSOMES AND DRUG DELIVERY con
- Page 476 and 477:
460 LIPOSOMES AND DRUG DELIVERY The
- Page 478 and 479:
462 LIPOSOMES AND DRUG DELIVERY in
- Page 480 and 481:
464 LIPOSOMES AND DRUG DELIVERY c a
- Page 482 and 483:
466 LIPOSOMES AND DRUG DELIVERY Eve
- Page 484 and 485:
468 LIPOSOMES AND DRUG DELIVERY inc
- Page 486 and 487:
470 LIPOSOMES AND DRUG DELIVERY wit
- Page 488 and 489:
472 LIPOSOMES AND DRUG DELIVERY i.v
- Page 490 and 491:
474 LIPOSOMES AND DRUG DELIVERY for
- Page 492 and 493:
476 LIPOSOMES AND DRUG DELIVERY bet
- Page 494 and 495:
478 LIPOSOMES AND DRUG DELIVERY cli
- Page 496 and 497:
480 LIPOSOMES AND DRUG DELIVERY lea
- Page 498 and 499:
482 LIPOSOMES AND DRUG DELIVERY Vag
- Page 500 and 501:
484 LIPOSOMES AND DRUG DELIVERY TAB
- Page 502 and 503:
486 LIPOSOMES AND DRUG DELIVERY thr
- Page 504 and 505:
488 LIPOSOMES AND DRUG DELIVERY TAB
- Page 506 and 507:
490 LIPOSOMES AND DRUG DELIVERY tar
- Page 508 and 509:
492 LIPOSOMES AND DRUG DELIVERY tha
- Page 510 and 511:
494 LIPOSOMES AND DRUG DELIVERY wit
- Page 512 and 513:
496 LIPOSOMES AND DRUG DELIVERY Pul
- Page 514 and 515:
498 LIPOSOMES AND DRUG DELIVERY whi
- Page 516 and 517:
500 LIPOSOMES AND DRUG DELIVERY Inc
- Page 518 and 519:
502 LIPOSOMES AND DRUG DELIVERY Per
- Page 520 and 521:
504 LIPOSOMES AND DRUG DELIVERY can
- Page 522 and 523:
506 LIPOSOMES AND DRUG DELIVERY Tum
- Page 524 and 525:
508 LIPOSOMES AND DRUG DELIVERY 25.
- Page 526 and 527:
510 LIPOSOMES AND DRUG DELIVERY 60.
- Page 528 and 529:
512 LIPOSOMES AND DRUG DELIVERY 97.
- Page 530 and 531:
514 LIPOSOMES AND DRUG DELIVERY 136
- Page 532 and 533:
516 LIPOSOMES AND DRUG DELIVERY 170
- Page 534 and 535:
518 LIPOSOMES AND DRUG DELIVERY 202
- Page 536 and 537:
520 LIPOSOMES AND DRUG DELIVERY 237
- Page 538 and 539:
522 LIPOSOMES AND DRUG DELIVERY 271
- Page 540 and 541:
524 LIPOSOMES AND DRUG DELIVERY 310
- Page 542 and 543:
526 LIPOSOMES AND DRUG DELIVERY 346
- Page 544 and 545:
528 LIPOSOMES AND DRUG DELIVERY 378
- Page 546 and 547:
530 LIPOSOMES AND DRUG DELIVERY 408
- Page 548 and 549:
532 LIPOSOMES AND DRUG DELIVERY 437
- Page 551 and 552:
5.4 BIODEGRADABLE NANOPARTICLES Sud
- Page 553 and 554:
NATURAL BIODEGRADABLE POLYMERIC NAN
- Page 555 and 556:
NATURAL BIODEGRADABLE POLYMERIC NAN
- Page 557 and 558:
NATURAL BIODEGRADABLE POLYMERIC NAN
- Page 559 and 560:
and drug loading of gliadin nanopar
- Page 561 and 562:
SYNTHETIC BIODEGRADABLE POLYMERIC N
- Page 563 and 564:
APPLICATIONS OF BIODEGRADABLE NANOP
- Page 565 and 566:
PHYSICOCHEMICAL CHARACTERIZATION OF
- Page 567 and 568:
TARGETING NANOPARTICLES BY SURFACE
- Page 569 and 570:
5.4.9 CONCLUSIONS Biodegradable nan
- Page 571 and 572:
REFERENCES 555 of a model protein (
- Page 573 and 574:
REFERENCES 557 65. Kaul , G. , and
- Page 575 and 576:
REFERENCES 559 99. Carino , G. P. ,
- Page 577 and 578:
REFERENCES 561 133. Na , K. , Lee ,
- Page 579 and 580:
REFERENCES 563 168. Freitas , C. ,
- Page 581 and 582:
5.5 RECOMBINANT SACCHAROMYCES CEREV
- Page 583 and 584:
Potential Medical Applications of B
- Page 585 and 586:
BIODRUG CONCEPT USING YEAST AS VECT
- Page 587 and 588:
BIODRUG CONCEPT USING YEAST AS VECT
- Page 589 and 590:
Cumulative ileal delivery of viable
- Page 591 and 592:
tine [30] . No signal was detectabl
- Page 593 and 594:
ORAL FORMULATION OF RECOMBINANT YEA
- Page 595 and 596:
Cumulative ileal delivery of viable
- Page 597 and 598:
ORAL FORMULATION OF RECOMBINANT YEA
- Page 599 and 600:
Viable yeasts released (% of initia
- Page 601 and 602:
Steidler et al. [47] have already d
- Page 603 and 604:
REFERENCES 587 23. Steidler , L. (
- Page 605 and 606:
REFERENCES 589 nant yeasts as novel
- Page 607 and 608:
5.6 NASAL DELIVERY OF PEPTIDE AND N
- Page 609 and 610:
fi rst - pass metabolism and gut -
- Page 611 and 612:
[6] . The nasal blood vessels can b
- Page 613 and 614:
FACTORS INFLUENCING NASAL DRUG ABSO
- Page 615 and 616:
5.6.3.6 Type of Delivery Device FAC
- Page 617 and 618:
FACTORS INFLUENCING NASAL DRUG ABSO
- Page 619 and 620:
FIGURE 4 ( a ) Optinose multidose l
- Page 621 and 622:
Dogs, sheep, and monkeys can be kep
- Page 623 and 624:
NASAL DELIVERY OF PEPTIDE AND HIGH-
- Page 625 and 626:
NASAL DELIVERY OF PEPTIDE AND HIGH-
- Page 627 and 628:
NASAL DELIVERY OF PEPTIDE AND HIGH-
- Page 629 and 630:
NASAL DELIVERY OF PEPTIDE AND HIGH-
- Page 631 and 632:
NASAL DELIVERY OF PEPTIDE AND HIGH-
- Page 633 and 634:
in solutions containing different c
- Page 635 and 636:
NASAL DELIVERY OF PEPTIDE AND HIGH-
- Page 637 and 638:
NASAL DELIVERY OF PEPTIDE AND HIGH-
- Page 639 and 640:
Morphine plasma concentration (nmol
- Page 641 and 642:
Concentration (μg/L) 1000 100 10 1
- Page 643 and 644:
NASAL DELIVERY OF NONPEPTIDE MOLECU
- Page 645 and 646:
NASAL DELIVERY OF NONPEPTIDE MOLECU
- Page 647 and 648:
OPTION FOR DELIVERY OF DRUGS TO CEN
- Page 649 and 650:
pseudostratifi ed epithelium compri
- Page 651 and 652:
NASAL DELIVERY OF VACCINES 635 Howe
- Page 653 and 654:
TABLE 5 Continued Delivery System/A
- Page 655 and 656:
REFERENCES 639 23. Hardy , J. G. ,
- Page 657 and 658:
REFERENCES 641 60. Collens , W. S.
- Page 659 and 660:
REFERENCES 643 97. Eden , S. ( 1979
- Page 661 and 662:
REFERENCES 645 comparison with oral
- Page 663 and 664:
REFERENCES 647 172. Sakane , T. , A
- Page 665 and 666:
REFERENCES 649 213. McCluskie , M.
- Page 667 and 668:
5.7 NASAL POWDER DRUG DELIVERY Jele
- Page 669 and 670:
NASAL DRY POWDER FORMULATIONS 653 t
- Page 671 and 672:
POLYMERS IN NASAL POWDER DELIVERY S
- Page 673 and 674:
POLYMERS IN NASAL POWDER DELIVERY S
- Page 675 and 676:
MICROSPHERES AS NASAL DRUG DELIVERY
- Page 677 and 678:
MICROSPHERES AS NASAL DRUG DELIVERY
- Page 679 and 680:
MICROSPHERES AS NASAL DRUG DELIVERY
- Page 681 and 682:
MICROSPHERES AS NASAL DRUG DELIVERY
- Page 683 and 684:
TOXICOLOGICAL CONSIDERATIONS 667 et
- Page 685 and 686:
Summary of Research Work on Nasal D
- Page 687 and 688:
Powder Formulation Preparation Meth
- Page 689 and 690:
Powder Formulation Preparation Meth
- Page 691 and 692:
REFERENCES 675 19. Van der Lubben ,
- Page 693 and 694:
REFERENCES 677 54. Witschi , C. , a
- Page 695 and 696:
REFERENCES 679 91. Varshosaz , J. ,
- Page 697:
REFERENCES 681 126. Jorissen , M. ,
- Page 700 and 701:
684 AEROSOL DRUG DELIVERY 5.8.8 5.8
- Page 702 and 703:
686 AEROSOL DRUG DELIVERY other sig
- Page 704 and 705:
688 AEROSOL DRUG DELIVERY are a num
- Page 706 and 707:
690 AEROSOL DRUG DELIVERY 5.8.5 MET
- Page 708 and 709:
692 AEROSOL DRUG DELIVERY TABLE 1 S
- Page 710 and 711:
694 AEROSOL DRUG DELIVERY valve siz
- Page 712 and 713:
696 AEROSOL DRUG DELIVERY Ferrule U
- Page 714 and 715:
698 AEROSOL DRUG DELIVERY 5.8.5.6 A
- Page 716 and 717:
700 AEROSOL DRUG DELIVERY 5.8.5.10
- Page 718 and 719:
702 AEROSOL DRUG DELIVERY low inter
- Page 720 and 721:
704 AEROSOL DRUG DELIVERY Transpare
- Page 722 and 723:
706 AEROSOL DRUG DELIVERY of the cl
- Page 724 and 725:
708 AEROSOL DRUG DELIVERY is achiev
- Page 726 and 727:
710 AEROSOL DRUG DELIVERY Aerosol g
- Page 728 and 729:
712 AEROSOL DRUG DELIVERY REFERENCE
- Page 730 and 731:
714 AEROSOL DRUG DELIVERY 42. Witek
- Page 732 and 733:
716 AEROSOL DRUG DELIVERY 81. Ohmor
- Page 734 and 735:
718 AEROSOL DRUG DELIVERY 119. Lewi
- Page 736 and 737:
720 AEROSOL DRUG DELIVERY 153. Terz
- Page 738 and 739:
722 AEROSOL DRUG DELIVERY 189. Sham
- Page 740 and 741:
724 AEROSOL DRUG DELIVERY 225. Newh
- Page 742 and 743:
726 AEROSOL DRUG DELIVERY 263. Dolo
- Page 745 and 746:
5.9 OCULAR DRUG DELIVERY Ilva D. Ru
- Page 747 and 748:
CHALLENGES IN OCULAR DRUG DELIVERY
- Page 749 and 750:
CHALLENGES IN OCULAR DRUG DELIVERY
- Page 751 and 752:
CHALLENGES IN OCULAR DRUG DELIVERY
- Page 753 and 754:
FORMULATION APPROACHES TO IMPROVE O
- Page 755 and 756:
FORMULATION APPROACHES TO IMPROVE O
- Page 757 and 758:
Drug Fluorescein FORMULATION APPROA
- Page 759 and 760:
FORMULATION APPROACHES TO IMPROVE O
- Page 761 and 762:
FORMULATION APPROACHES TO IMPROVE O
- Page 763 and 764:
FORMULATION APPROACHES TO IMPROVE O
- Page 765 and 766:
FORMULATION APPROACHES TO IMPROVE O
- Page 767 and 768:
FORMULATION APPROACHES TO IMPROVE O
- Page 769 and 770:
is characterized by a transient ove
- Page 771 and 772:
REFERENCES 755 11. Klyce , S. D. ,
- Page 773 and 774:
REFERENCES 757 53. Saettone , M. F.
- Page 775 and 776:
REFERENCES 759 87. Lin , H. R. , an
- Page 777 and 778:
REFERENCES 761 122. Durrani , A. M.
- Page 779 and 780:
REFERENCES 763 158. Weyenberg , W.
- Page 781 and 782:
REFERENCES 765 of various ophthalmi
- Page 783:
REFERENCES 767 233. Grass , G. M. ,
- Page 786 and 787:
770 MICROEMULSIONS AS DRUG DELIVERY
- Page 788 and 789:
772 MICROEMULSIONS AS DRUG DELIVERY
- Page 790 and 791:
774 MICROEMULSIONS AS DRUG DELIVERY
- Page 792 and 793:
776 MICROEMULSIONS AS DRUG DELIVERY
- Page 794 and 795:
778 MICROEMULSIONS AS DRUG DELIVERY
- Page 796 and 797:
780 MICROEMULSIONS AS DRUG DELIVERY
- Page 798 and 799:
782 MICROEMULSIONS AS DRUG DELIVERY
- Page 800 and 801:
784 MICROEMULSIONS AS DRUG DELIVERY
- Page 802 and 803:
786 MICROEMULSIONS AS DRUG DELIVERY
- Page 804 and 805:
788 MICROEMULSIONS AS DRUG DELIVERY
- Page 806 and 807:
790 MICROEMULSIONS AS DRUG DELIVERY
- Page 808 and 809:
792 MICROEMULSIONS AS DRUG DELIVERY
- Page 810 and 811:
794 TRANSDERMAL DRUG DELIVERY 5.11.
- Page 812 and 813:
796 TRANSDERMAL DRUG DELIVERY conta
- Page 814 and 815:
798 TRANSDERMAL DRUG DELIVERY 5.11.
- Page 816 and 817:
800 TRANSDERMAL DRUG DELIVERY FIGUR
- Page 818 and 819:
802 TRANSDERMAL DRUG DELIVERY a rat
- Page 820 and 821:
804 TRANSDERMAL DRUG DELIVERY passi
- Page 822 and 823:
806 TRANSDERMAL DRUG DELIVERY 19. E
- Page 825 and 826:
5.12 VAGINAL DRUG DELIVERY Jos é d
- Page 827 and 828:
Therefore, this chapter discusses t
- Page 829 and 830:
THE HUMAN VAGINA 813 thetic innerva
- Page 831 and 832:
THE HUMAN VAGINA 815 the upper repr
- Page 833 and 834:
THE HUMAN VAGINA 817 tant in the re
- Page 835 and 836:
GENERAL FEATURES OF VAGINAL DRUG DE
- Page 837 and 838:
Although this strategy may enhance
- Page 839 and 840:
VAGINAL DRUG DELIVERY SYSTEMS 823 a
- Page 841 and 842:
VAGINAL DRUG DELIVERY SYSTEMS 825 f
- Page 843 and 844:
VAGINAL DRUG DELIVERY SYSTEMS 827 f
- Page 845 and 846:
Amount released, μg/day 800 700 60
- Page 847 and 848:
VAGINAL DRUG DELIVERY SYSTEMS 831 5
- Page 849 and 850:
TABLE 4 Examples of Mucoadhesive Po
- Page 851 and 852:
VAGINAL DRUG DELIVERY SYSTEMS 835 i
- Page 853 and 854:
PHARMACEUTICAL EVALUATION OF VAGINA
- Page 855 and 856:
PHARMACEUTICAL EVALUATION OF VAGINA
- Page 857 and 858:
PHARMACEUTICAL EVALUATION OF VAGINA
- Page 859 and 860:
CLINICAL USAGE AND POTENTIAL OF VAG
- Page 861 and 862:
CLINICAL USAGE AND POTENTIAL OF VAG
- Page 863 and 864:
CLINICAL USAGE AND POTENTIAL OF VAG
- Page 865 and 866:
CLINICAL USAGE AND POTENTIAL OF VAG
- Page 867 and 868:
CLINICAL USAGE AND POTENTIAL OF VAG
- Page 869 and 870:
TABLE 6 Selected Drugs Administered
- Page 871 and 872:
Selected Veterinary Vaginal Drug De
- Page 873 and 874:
REFERENCES 857 Much work remains to
- Page 875 and 876:
REFERENCES 859 33. Hocini , H. , Ba
- Page 877 and 878:
REFERENCES 861 70. Kristmundsdottir
- Page 879 and 880:
REFERENCES 863 105. McClelland , R.
- Page 881 and 882:
REFERENCES 865 141. Kuyoh , M. A. ,
- Page 883 and 884:
REFERENCES 867 178. Ondracek , J. ,
- Page 885 and 886:
REFERENCES 869 212. Bagga , R. , Ra
- Page 887 and 888:
REFERENCES 871 244. International W
- Page 889 and 890:
REFERENCES 873 276. Mor , E. , Saad
- Page 891 and 892:
REFERENCES 875 310. Saltzman , W. M
- Page 893 and 894:
REFERENCES 877 vaginal microbicides
- Page 895:
SECTION 6 TABLET PRODUCTION
- Page 898 and 899:
882 PHARMACEUTICAL PREFORMULATION 6
- Page 900 and 901:
884 PHARMACEUTICAL PREFORMULATION E
- Page 902 and 903:
886 PHARMACEUTICAL PREFORMULATION T
- Page 904 and 905:
888 PHARMACEUTICAL PREFORMULATION T
- Page 906 and 907:
890 PHARMACEUTICAL PREFORMULATION T
- Page 908 and 909:
892 PHARMACEUTICAL PREFORMULATION p
- Page 910 and 911:
894 PHARMACEUTICAL PREFORMULATION T
- Page 912 and 913:
896 PHARMACEUTICAL PREFORMULATION T
- Page 914 and 915:
898 PHARMACEUTICAL PREFORMULATION l
- Page 916 and 917:
900 PHARMACEUTICAL PREFORMULATION T
- Page 918 and 919:
902 PHARMACEUTICAL PREFORMULATION s
- Page 920 and 921:
904 PHARMACEUTICAL PREFORMULATION C
- Page 922 and 923:
906 PHARMACEUTICAL PREFORMULATION p
- Page 924 and 925:
908 PHARMACEUTICAL PREFORMULATION C
- Page 926 and 927:
910 PHARMACEUTICAL PREFORMULATION T
- Page 928 and 929:
912 PHARMACEUTICAL PREFORMULATION D
- Page 930 and 931:
914 PHARMACEUTICAL PREFORMULATION C
- Page 932 and 933:
916 PHARMACEUTICAL PREFORMULATION u
- Page 934 and 935:
918 PHARMACEUTICAL PREFORMULATION d
- Page 936 and 937:
920 PHARMACEUTICAL PREFORMULATION q
- Page 938 and 939:
922 PHARMACEUTICAL PREFORMULATION w
- Page 940 and 941:
924 PHARMACEUTICAL PREFORMULATION C
- Page 942 and 943:
926 PHARMACEUTICAL PREFORMULATION T
- Page 944 and 945:
928 PHARMACEUTICAL PREFORMULATION a
- Page 946 and 947:
930 PHARMACEUTICAL PREFORMULATION a
- Page 949 and 950:
6.2 ROLE OF PREFORMULATION IN DEVEL
- Page 951 and 952:
maceutics ” documentation forms a
- Page 953 and 954:
e altered without any change in the
- Page 955 and 956:
Solubility Heat flow II I T m,II Ts
- Page 957 and 958:
TABLE 2 Techniques to Characterize
- Page 959 and 960:
Percent weight loss Heat flow (a) (
- Page 961 and 962:
Heat flow Intensity 100 50 Endother
- Page 963 and 964:
Change in mass (%) PHYSICAL/BULK CH
- Page 965 and 966:
6.2.2.6 Powder Flow and Compressibi
- Page 967 and 968:
Solvent required for 1 gm of solid
- Page 969 and 970:
⎧ 100 ⎪ Ka 1+10 Percent ionized
- Page 971 and 972:
Percent Ionization Percent Ionizati
- Page 973 and 974:
SOLUBILITY CHARACTERISTICS 957 para
- Page 975 and 976:
SOLUBILITY CHARACTERISTICS 959 of p
- Page 977 and 978:
Dissolution studies pH solubility p
- Page 979 and 980:
High Permeability Low SOLUBILITY CH
- Page 981 and 982:
is evident form the fact [51] that
- Page 983 and 984:
independent of initial drug concent
- Page 985 and 986:
STABILITY CHARACTERISTICS 969 drug
- Page 987 and 988:
6.2.5 CONCLUSIONS Preformulation te
- Page 989 and 990:
REFERENCES 973 16. Huang , L. , and
- Page 991:
54. 55. 56. REFERENCES 975 IFAMA (
- Page 994 and 995:
978 TABLET DESIGN 6.3.9.3 Case Stud
- Page 996 and 997:
980 TABLET DESIGN Another reason, t
- Page 998 and 999:
982 TABLET DESIGN E: This ensures t
- Page 1000 and 1001:
984 TABLET DESIGN substance would b
- Page 1002 and 1003:
986 TABLET DESIGN Plasticity 1.0 0.
- Page 1004 and 1005:
988 TABLET DESIGN Drug release (%)
- Page 1006 and 1007:
990 TABLET DESIGN FIGURE 9 Dissolut
- Page 1008 and 1009:
992 TABLET DESIGN TABLE 2 Number 1
- Page 1010 and 1011:
994 TABLET DESIGN Formulation Table
- Page 1012 and 1013:
996 TABLET DESIGN TABLE 4 Theophyll
- Page 1014 and 1015:
998 TABLET DESIGN often very fi ne,
- Page 1016 and 1017:
1000 TABLE 6 Formulations by Wet Gr
- Page 1018 and 1019:
1002 TABLET DESIGN 6.3.6.2 FIGURE 1
- Page 1020 and 1021:
1004 TABLET DESIGN TABLE 9 Papaveri
- Page 1022 and 1023:
1006 TABLET DESIGN TABLE 12 Weight
- Page 1024 and 1025:
1008 TABLET DESIGN (3.179 and 4.500
- Page 1026 and 1027:
1010 TABLET DESIGN TABLE 14 Run Ord
- Page 1028 and 1029:
1012 TABLET DESIGN Regression coeff
- Page 1030 and 1031:
1014 TABLET DESIGN to being the out
- Page 1032 and 1033:
1016 TABLET DESIGN Mechanical Prope
- Page 1034 and 1035:
1018 TABLET DESIGN drawbacks (unsaf
- Page 1036 and 1037:
1020 TABLET DESIGN TABLE 16 Basic F
- Page 1038 and 1039:
1022 TABLET DESIGN PEG (e.g., Macro
- Page 1040 and 1041:
1024 TABLET DESIGN 0.45% 0.40% 0.35
- Page 1042 and 1043:
1026 TABLET DESIGN FIGURE 34 Granul
- Page 1044 and 1045:
1028 TABLET DESIGN TABLE 19 SEPIFIL
- Page 1046 and 1047:
1030 TABLET DESIGN (c) Blistering D
- Page 1048 and 1049:
1032 TABLET DESIGN % Dextromethorph
- Page 1050 and 1051:
1034 TABLET DESIGN (a) (b) 500 μm
- Page 1052 and 1053:
1036 TABLET DESIGN threshold p c1 a
- Page 1054 and 1055:
1038 TABLET DESIGN TABLE 24 Composi
- Page 1056 and 1057:
1040 TABLET DESIGN Korsmeyer - Pepp
- Page 1058 and 1059:
1042 TABLET DESIGN % Water uptake/d
- Page 1060 and 1061:
1044 TABLET DESIGN FIGURE 47 Temper
- Page 1062 and 1063:
1046 TABLET DESIGN REFERENCES 1. Un
- Page 1064 and 1065:
1048 TABLET DESIGN 34. Congreve , M
- Page 1066 and 1067:
1050 TABLET DESIGN 73. Miranda , A.
- Page 1069 and 1070:
6.4 TABLET PRODUCTION SYSTEMS Katha
- Page 1071 and 1072:
PHYSICS OF TABLET FORMATION 1055 en
- Page 1073 and 1074:
Tablet production systems can be op
- Page 1075 and 1076:
TABLETING MACHINES 1059 Eccentric T
- Page 1077 and 1078:
TABLETING MACHINES 1061 In Figure 4
- Page 1079 and 1080:
TABLETING MACHINE SIMULATORS (COMPA
- Page 1081 and 1082:
TABLETING MACHINE SIMULATORS (COMPA
- Page 1083 and 1084:
However, for mechanical compaction
- Page 1085 and 1086:
INSTRUMENTATION OF TABLETING MACHIN
- Page 1087 and 1088:
Force (kN) FIGURE 13 ANALYSIS OF TA
- Page 1089 and 1090:
TABLE 4 Source Emschermann [91] (Fi
- Page 1091 and 1092:
TABLE 6 Parameters Directly Deduced
- Page 1093 and 1094:
TABLE 7 Parameters Calculated from
- Page 1095 and 1096:
ANALYSIS OF TABLETING PROCESS 1079
- Page 1097 and 1098:
TABLE 8 Source 3D model [143, 144,
- Page 1099 and 1100:
6.4.11 SPECIAL ACCESSORIES OF TABLE
- Page 1101 and 1102:
usually tends to vary. However, the
- Page 1103 and 1104:
ing at punches and dies. Low produc
- Page 1105 and 1106:
REFERENCES 1089 24. Elamin , A. , S
- Page 1107 and 1108:
REFERENCES 1091 64. Yeh , C. , Alta
- Page 1109 and 1110:
REFERENCES 1093 100. Armstrong , N.
- Page 1111 and 1112:
REFERENCES 1095 140. Kuentz , M. ,
- Page 1113 and 1114:
REFERENCES 1097 178. Hauschild , K.
- Page 1115 and 1116:
6.5 CONTROLLED RELEASE OF DRUGS FRO
- Page 1117 and 1118:
INTRODUCTION 1101 ings, and their e
- Page 1119 and 1120:
needed to circulate them [57] . Typ
- Page 1121 and 1122:
APPLICATIONS 1105 stream. During dr
- Page 1123 and 1124:
APPLICATIONS 1107 TABLE 1 Theophyll
- Page 1125 and 1126:
APPLICATIONS 1109 FIGURE 1 Release
- Page 1127 and 1128:
(a) (b) APPLICATIONS 1111 FIGURE 3
- Page 1129 and 1130:
APPLICATIONS 1113 FIGURE 5 Ternary
- Page 1131 and 1132:
APPLICATIONS 1115 of the drug, dC/d
- Page 1133 and 1134:
APPLICATIONS 1117 FIGURE 9 Ternary
- Page 1135 and 1136:
Release rate (mg/min) 0.40 0.30 0.2
- Page 1137 and 1138:
REFERENCES REFERENCES 1121 1. Lange
- Page 1139 and 1140:
REFERENCES 1123 37. Lin , Y - K. ,
- Page 1141 and 1142:
REFERENCES 1125 73. St - Onge , L.
- Page 1143 and 1144:
REFERENCES 1127 107. Lai , J. - Y.
- Page 1145 and 1146:
APPENDIX 1129 FIGURE A3 Release of
- Page 1147 and 1148:
APPENDIX 1131 FIGURE A7 Release of
- Page 1149 and 1150:
6.6 TABLET COMPRESSION Helton M. M.
- Page 1151 and 1152:
during compression, which will also
- Page 1153 and 1154:
of powder mixtures is usually chara
- Page 1155 and 1156:
lose in combination with either tal
- Page 1157 and 1158:
Due to the signifi cant nonlinearit
- Page 1159 and 1160:
EQUIPMENT FOR TABLET COMPRESSION 11
- Page 1161 and 1162:
Product Fill cam (mm) column height
- Page 1163 and 1164:
TABLET PRESS TOOLING 1147 TABLE 1 T
- Page 1165 and 1166:
TABLET PRESS TOOLING 1149 7. Tungst
- Page 1167 and 1168:
FIGURE 6 Tooling standards confi gu
- Page 1169 and 1170:
6.6.7 TABLE ENGRAVING Engraving is
- Page 1171 and 1172:
Shallow and standard concave tablet
- Page 1173 and 1174:
is the most popular bisect confi gu
- Page 1175 and 1176:
PROBLEMS DURING TABLET MANUFACTURIN
- Page 1177 and 1178:
thus reducing a potential variation
- Page 1179:
REFERENCES 1163 27. Train , D. ( 19
- Page 1182 and 1183:
1166 EFFECTS OF GRINDING IN PHARMAC
- Page 1184 and 1185:
1168 EFFECTS OF GRINDING IN PHARMAC
- Page 1186 and 1187:
1170 EFFECTS OF GRINDING IN PHARMAC
- Page 1188 and 1189:
1172 EFFECTS OF GRINDING IN PHARMAC
- Page 1190 and 1191:
1174 EFFECTS OF GRINDING IN PHARMAC
- Page 1192 and 1193:
1176 EFFECTS OF GRINDING IN PHARMAC
- Page 1194 and 1195:
1178 EFFECTS OF GRINDING IN PHARMAC
- Page 1196 and 1197:
1180 EFFECTS OF GRINDING IN PHARMAC
- Page 1198 and 1199:
1182 EFFECTS OF GRINDING IN PHARMAC
- Page 1200 and 1201:
1184 EFFECTS OF GRINDING IN PHARMAC
- Page 1202 and 1203:
1186 EFFECTS OF GRINDING IN PHARMAC
- Page 1204 and 1205:
1188 EFFECTS OF GRINDING IN PHARMAC
- Page 1206 and 1207:
1190 EFFECTS OF GRINDING IN PHARMAC
- Page 1208 and 1209:
1192 ORAL EXTENDED-RELEASE FORMULAT
- Page 1210 and 1211:
1194 ORAL EXTENDED-RELEASE FORMULAT
- Page 1212 and 1213:
1196 ORAL EXTENDED-RELEASE FORMULAT
- Page 1214 and 1215:
1198 ORAL EXTENDED-RELEASE FORMULAT
- Page 1216 and 1217:
1200 ORAL EXTENDED-RELEASE FORMULAT
- Page 1218 and 1219:
1202 ORAL EXTENDED-RELEASE FORMULAT
- Page 1220 and 1221:
1204 ORAL EXTENDED-RELEASE FORMULAT
- Page 1222 and 1223:
1206 ORAL EXTENDED-RELEASE FORMULAT
- Page 1224 and 1225:
1208 ORAL EXTENDED-RELEASE FORMULAT
- Page 1226 and 1227:
1210 ORAL EXTENDED-RELEASE FORMULAT
- Page 1228 and 1229:
1212 ORAL EXTENDED-RELEASE FORMULAT
- Page 1230 and 1231:
1214 ORAL EXTENDED-RELEASE FORMULAT
- Page 1232 and 1233:
1216 ORAL EXTENDED-RELEASE FORMULAT
- Page 1234 and 1235:
1218 ORAL EXTENDED-RELEASE FORMULAT
- Page 1236 and 1237:
1220 ORAL EXTENDED-RELEASE FORMULAT
- Page 1238 and 1239:
1222 ORAL EXTENDED-RELEASE FORMULAT
- Page 1241 and 1242:
7.1 CYCLODEXTRIN - BASED NANOMATERI
- Page 1243 and 1244:
OH (3) OH (2) HOCH 2 HOCH 2 O O OH
- Page 1245 and 1246:
APPLICATIONS OF CYCLODEXTRINS IN NA
- Page 1247 and 1248:
Percentage of release 100 90 80 70
- Page 1249 and 1250:
APPLICATIONS OF CYCLODEXTRINS IN NA
- Page 1251 and 1252:
APPLICATIONS OF CYCLODEXTRINS IN NA
- Page 1253 and 1254:
APPLICATIONS OF CYCLODEXTRINS IN NA
- Page 1255 and 1256:
O APPLICATIONS OF CYCLODEXTRINS IN
- Page 1257 and 1258:
β - CDC6 loaded with the model dru
- Page 1259 and 1260:
REFERENCES 1243 13. Stella , V. J.
- Page 1261 and 1262:
REFERENCES 1245 49. Dodziuk , H. ,
- Page 1263:
REFERENCES 1247 81. Memi şo ğlu -
- Page 1266 and 1267:
1250 NANOTECHNOLOGY IN PHARMACEUTIC
- Page 1268 and 1269:
1252 NANOTECHNOLOGY IN PHARMACEUTIC
- Page 1270 and 1271:
1254 NANOTECHNOLOGY IN PHARMACEUTIC
- Page 1272 and 1273:
1256 NANOTECHNOLOGY IN PHARMACEUTIC
- Page 1274 and 1275:
1258 NANOTECHNOLOGY IN PHARMACEUTIC
- Page 1276 and 1277:
1260 NANOTECHNOLOGY IN PHARMACEUTIC
- Page 1278 and 1279:
1262 NANOTECHNOLOGY IN PHARMACEUTIC
- Page 1280 and 1281:
1264 NANOTECHNOLOGY IN PHARMACEUTIC
- Page 1282 and 1283:
1266 NANOTECHNOLOGY IN PHARMACEUTIC
- Page 1284 and 1285:
1268 NANOTECHNOLOGY IN PHARMACEUTIC
- Page 1286 and 1287:
1270 NANOTECHNOLOGY IN PHARMACEUTIC
- Page 1288 and 1289:
1272 NANOTECHNOLOGY IN PHARMACEUTIC
- Page 1290 and 1291:
1274 NANOTECHNOLOGY IN PHARMACEUTIC
- Page 1292 and 1293:
1276 NANOTECHNOLOGY IN PHARMACEUTIC
- Page 1294 and 1295:
1278 NANOTECHNOLOGY IN PHARMACEUTIC
- Page 1296 and 1297:
1280 NANOTECHNOLOGY IN PHARMACEUTIC
- Page 1298 and 1299:
1282 NANOTECHNOLOGY IN PHARMACEUTIC
- Page 1300 and 1301:
1284 NANOTECHNOLOGY IN PHARMACEUTIC
- Page 1302 and 1303:
1286 NANOTECHNOLOGY IN PHARMACEUTIC
- Page 1304 and 1305:
1288 NANOTECHNOLOGY IN PHARMACEUTIC
- Page 1306 and 1307:
1290 PHARMACEUTICAL NANOSYSTEMS 7.3
- Page 1308 and 1309:
1292 PHARMACEUTICAL NANOSYSTEMS TAB
- Page 1310 and 1311:
1294 PHARMACEUTICAL NANOSYSTEMS TAB
- Page 1312 and 1313:
1296 PHARMACEUTICAL NANOSYSTEMS Lam
- Page 1314 and 1315:
1298 PHARMACEUTICAL NANOSYSTEMS 7.3
- Page 1316 and 1317:
1300 PHARMACEUTICAL NANOSYSTEMS sta
- Page 1318 and 1319:
1302 PHARMACEUTICAL NANOSYSTEMS TAB
- Page 1320 and 1321:
1304 PHARMACEUTICAL NANOSYSTEMS har
- Page 1322 and 1323:
1306 PHARMACEUTICAL NANOSYSTEMS TAB
- Page 1324 and 1325:
1308 PHARMACEUTICAL NANOSYSTEMS Sur
- Page 1326 and 1327:
1310 PHARMACEUTICAL NANOSYSTEMS Aut
- Page 1328 and 1329:
1312 PHARMACEUTICAL NANOSYSTEMS ®
- Page 1330 and 1331:
1314 PHARMACEUTICAL NANOSYSTEMS 55.
- Page 1332 and 1333:
1316 PHARMACEUTICAL NANOSYSTEMS 100
- Page 1334 and 1335:
1318 PHARMACEUTICAL NANOSYSTEMS 147
- Page 1336 and 1337:
1320 PHARMACEUTICAL NANOSYSTEMS 191
- Page 1338 and 1339:
1322 PHARMACEUTICAL NANOSYSTEMS 230
- Page 1340 and 1341:
1324 PHARMACEUTICAL NANOSYSTEMS Nan
- Page 1343 and 1344:
7.4 OIL - IN - WATER NANOSIZED EMUL
- Page 1345 and 1346:
GENERATIONS OF OIL-IN-WATER NANOSIZ
- Page 1347 and 1348:
GENERATIONS OF OIL-IN-WATER NANOSIZ
- Page 1349 and 1350:
GENERATIONS OF OIL-IN-WATER NANOSIZ
- Page 1351 and 1352:
GENERATIONS OF OIL-IN-WATER NANOSIZ
- Page 1353 and 1354:
GENERATIONS OF OIL-IN-WATER NANOSIZ
- Page 1355 and 1356:
GENERATIONS OF OIL-IN-WATER NANOSIZ
- Page 1357 and 1358:
DRUG-FREE/LOADED OIL-IN-WATER NANOS
- Page 1359 and 1360:
EXCIPIENT INCLUSION: OIL-IN-WATER N
- Page 1361 and 1362:
EXCIPIENT INCLUSION: OIL-IN-WATER N
- Page 1363 and 1364:
MEDICAL APPLICATIONS OF OIL-IN-WATE
- Page 1365 and 1366:
MEDICAL APPLICATIONS OF OIL-IN-WATE
- Page 1367 and 1368:
MEDICAL APPLICATIONS OF OIL-IN-WATE
- Page 1369 and 1370:
MEDICAL APPLICATIONS OF OIL-IN-WATE
- Page 1371 and 1372:
evaluation of the lipid hydroperoxi
- Page 1373 and 1374:
REFERENCES 1357 21. Ott , G. , Sing
- Page 1375 and 1376:
REFERENCES 1359 57. Harris , J. M.
- Page 1377 and 1378:
REFERENCES 1361 94. Kim , Y. J. , K
- Page 1379 and 1380:
REFERENCES 1363 131. Swietlikowska
- Page 1381 and 1382:
REFERENCES 1365 167. Acheampong , A
- Page 1383 and 1384:
INDEX Abortifacients, 850 Acyclovir
- Page 1385 and 1386:
Lung cancer, 497-502 Lung toxicity,