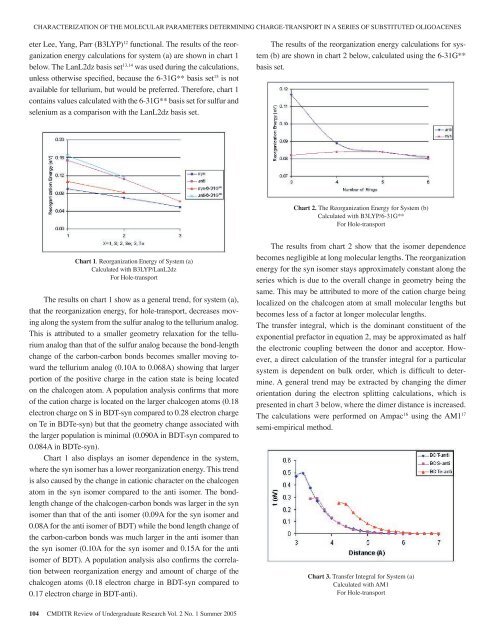
CHARACTERIZATION OF THE MOLECULAR PARAMETERS DETERMINING CHARGE-TRANSPORT IN A SERIES OF SUBSTITUTED OLIGOACENESeter Lee, Yang, Parr (B3LYP) 12 functional. The results <strong>of</strong> the reorganizationenergy calculations for system (a) are shown in chart 1below. The LanL2dz basis set 13,14 was used during the calculations,unless otherwise specified, because the 6-31G** basis set 15 is notavailable for tellurium, but would be preferred. Therefore, chart 1contains values calculated with the 6-31G** basis set for sulfur andselenium as a comparison with the LanL2dz basis set.The results <strong>of</strong> the reorganization energy calculations for system(b) are shown in chart 2 below, calculated using the 6-31G**basis set.Chart 2. The Reorganization Energy for System (b)Calculated with B3LYP/6-31G**For Hole-transportChart 1. Reorganization Energy <strong>of</strong> System (a)Calculated with B3LYP/LanL2dzFor Hole-transportThe results on chart 1 show as a general trend, for system (a),that the reorganization energy, for hole-transport, decreases movingalong the system from the sulfur analog to the tellurium analog.This is attributed to a smaller geometry relaxation for the telluriumanalog than that <strong>of</strong> the sulfur analog because the bond-lengthchange <strong>of</strong> the carbon-carbon bonds becomes smaller moving towardthe tellurium analog (0.10A to 0.068A) showing that largerportion <strong>of</strong> the positive charge in the cation state is being locatedon the chalcogen atom. A population analysis confirms that more<strong>of</strong> the cation charge is located on the larger chalcogen atoms (0.18electron charge on S in BDT-syn compared to 0.28 electron chargeon Te in BDTe-syn) but that the geometry change associated withthe larger population is minimal (0.090A in BDT-syn compared to0.084A in BDTe-syn).Chart 1 also displays an isomer dependence in the system,where the syn isomer has a lower reorganization energy. This trendis also caused by the change in cationic character on the chalcogenatom in the syn isomer compared to the anti isomer. The bondlengthchange <strong>of</strong> the chalcogen-carbon bonds was larger in the synisomer than that <strong>of</strong> the anti isomer (0.09A for the syn isomer and0.08A for the anti isomer <strong>of</strong> BDT) while the bond length change <strong>of</strong>the carbon-carbon bonds was much larger in the anti isomer thanthe syn isomer (0.10A for the syn isomer and 0.15A for the antiisomer <strong>of</strong> BDT). A population analysis also confirms the correlationbetween reorganization energy and amount <strong>of</strong> charge <strong>of</strong> thechalcogen atoms (0.18 electron charge in BDT-syn compared to0.17 electron charge in BDT-anti).The results from chart 2 show that the isomer dependencebecomes negligible at long molecular lengths. The reorganizationenergy for the syn isomer stays approximately constant along theseries which is due to the overall change in geometry being thesame. This may be attributed to more <strong>of</strong> the cation charge beinglocalized on the chalcogen atom at small molecular lengths butbecomes less <strong>of</strong> a factor at longer molecular lengths.The transfer integral, which is the dominant constituent <strong>of</strong> theexponential prefactor in equation 2, may be approximated as halfthe electronic coupling between the donor and acceptor. However,a direct calculation <strong>of</strong> the transfer integral for a particularsystem is dependent on bulk order, which is difficult to determine.A general trend may be extracted by changing the dimerorientation during the electron splitting calculations, which ispresented in chart 3 below, where the dimer distance is increased.The calculations were performed on Ampac 16 using the AM1 17semi-empirical method.Chart 3. Transfer Integral for System (a)Calculated with AM1For Hole-transport104 CMDITR Review <strong>of</strong> Undergraduate Research Vol. 2 No. 1 Summer <strong>2005</strong>
SNOEBERGERThe roll-over in the plots in chart 3 occur at approximatelytwice the vanderwaals radius <strong>of</strong> the chalcogen atom, whichis 3.7A for the sulfur analog, 4.0A for the selenium analog, and4.4A for the tellurium analog. The results <strong>of</strong> chart 3 show thatthe tellurium analog has a larger transfer integral at larger dimerdistances than that <strong>of</strong> the selenium and sulfur analogs, which isconsistent with the idea that a larger atomic radii will result ina larger transfer integral. However, this is not seen between thesulfur and selenium analogs which suggests that the interactionbetween the carbon orbitals dominant the electron splitting.The current work shows that including large chalcogen atomsin hole-transport materials results in desirably low reorganizationenergies and that the smaller molecules show an isomerdependence, which is more pronounced with the smaller chalcogenatoms. These systems are interesting for charge-transport andsynthesis is recommended.ACKNOWLEDGEMENTSResearch support is gratefully acknowledged from the NationalScience Foundation Center on Materials and Devices forInformation Technology Research (CMDITR), DMR-0120967.REFERENCES1. O.D. Jurchescu, J. Baas, T.T.M. Palstra, Appl. Phys. Lett. 84.3061-3063. (2004)2. J.H. Schon, Appl. Phys. Lett. 79. 4163-4162. (2001).3. M. Yamada, I. Ikemote, H. Kuroda, Bull. Chem. Soc Jpn.61.1057. (1988).4. O. Kwon, et. al. J. Chem. Phys. 120, 8186-8194. (2004).5. J.G. Laquindanum, H.E. Katz, A.J. Lovinger, J. Am. Chem.Soc. 120. 664-672. (1998).6. K.Takimiya, Y. Kunugi, Y, Konda, N. Niihara, T. Otsubo. J.Am. Chem. Soc. 126. 5084-5085. (2004).7. V. Coropceanu, J.M. Andre, M. Malagoli, J.L. Bredas. TheorChem Acc. 110. 59-69. (2003)8. M. Malagoli, V. Coropceanu, D.A. da Silva Filho, J.L. Bredas.J. Chem. Phys. 120, 7490-7496. (2004).9. M.J. Frisch, et al. Gaussian98, Revision A. 11, Gaussian, Incorporated:Wallingford, CT, 1998.10. P. Hohenberg and W. Kohn, Physical Review 136, B864-B871 (1964).11. R.G. Parr and W. Yang, Density-functional theory <strong>of</strong> atomsand molecules, Oxford Univ. Press: Oxford, (1989).12. A.D. Becke, J. Chem. Phys. 98 5648 (1993).13. T. H. Dunning, Jr. and P. J. Hay, in Modern Theoretical Chemistry,Ed. H. F. Schaefer, III, Plenum: New York, 1-28, (1976).14. P.J. Hay and W.R. Wadt, J. Chem. Phys. 82, 270 (1985).15. R. Ditchfield, W.J. Hehre and J.A. Pople, J. Chem. Phys. 54,724 (1971).16. AMPAC 8, © 1992-2004 Semichem, Inc. PO Box 1649,Shawnee, KS 66222.17. M.J.S. Dewar, E.G. Zoebisch, E.F. Healy, J.J.P. Stewart. J.Am. Chem. Soc. 107. 3902-3909. (1985).CMDITR Review <strong>of</strong> Undergraduate Research Vol. 2 No. 1 Summer <strong>2005</strong> 105
- Page 2 and 3:
The material is based upon work sup
- Page 4 and 5:
TABLE OF CONTENTSSynthesis of Dendr
- Page 6 and 7:
6 CMDITR Review of Undergraduate Re
- Page 8 and 9:
SYNTHESIS OF DENDRIMER BUILDING BLO
- Page 10 and 11:
throughout the work period. Five su
- Page 12 and 13:
12 CMDITR Review of Undergraduate R
- Page 14 and 15:
BARIUM TITANATE DOPED SOL-GEL FOR E
- Page 16 and 17:
BARIUM TITANATE DOPED SOL-GEL FOR E
- Page 18 and 19:
SYNTHESIS OF NORBORNENE MONOMER OF
- Page 20:
20 CMDITR Review of Undergraduate R
- Page 23 and 24:
using different reaction conditions
- Page 25 and 26:
Synthesis of Nonlinear Optical-Acti
- Page 27 and 28:
quality of the XRD structures wasca
- Page 29 and 30:
Behavioral Properties of Colloidal
- Page 32 and 33:
Transmission electron microscopy ha
- Page 34 and 35:
34 CMDITR Review of Undergraduate R
- Page 36 and 37:
areorient themselves with the elect
- Page 38 and 39:
Fabry-Perot modulators with electro
- Page 40 and 41:
40 CMDITR Review of Undergraduate R
- Page 42 and 43:
QUANTIZED HAMILTON DYNAMICS APPLIED
- Page 44 and 45:
44 CMDITR Review of Undergraduate R
- Page 46 and 47:
INVESTIGATING NEW CLADDING AND CORE
- Page 48 and 49:
Dr. Robert NorwoodChris DeRoseAmir
- Page 50 and 51:
SYNTHESIS OF TPD-BASED COMPOUNDS FO
- Page 52 and 53:
SYNTHESIS OF TPD-BASED COMPOUNDS FO
- Page 54 and 55: OPTIMIZING HYBRID WAVEGUIDESpropaga
- Page 56 and 57: At closer spaces the second undesir
- Page 58 and 59: SYNTHESIS AND ANALYSIS OF THIOL-STA
- Page 60 and 61: 60 CMDITR Review of Undergraduate R
- Page 62 and 63: QUINOXALINE-CONTAINING POLYFLUORENE
- Page 64 and 65: QUINOXALINE-CONTAINING POLYFLUORENE
- Page 66 and 67: 66 CMDITR Review of Undergraduate R
- Page 68 and 69: SYNTHESIS OF DENDRON-FUNCTIONALIZED
- Page 70 and 71: 70 CMDITR Review of Undergraduate R
- Page 72 and 73: BUILDING AN OPTICAL OXIMETER TO MEA
- Page 74 and 75: 74 CMDITR Review of Undergraduate R
- Page 76 and 77: 76 CMDITR Review of Undergraduate R
- Page 78 and 79: TOWARD MOLECULAR RESOLUTION C-AFM W
- Page 80 and 81: TOWARD MOLECULAR RESOLUTION C-AFM W
- Page 82 and 83: SYNTHESIS AND CHARACTERIZATION OF E
- Page 84 and 85: My name is Aaron Montgomery and I a
- Page 86 and 87: 1,1-DIPHENYL-2,3,4,5-TETRAKIS(9,9-D
- Page 88 and 89: 1,1-DIPHENYL-2,3,4,5-TETRAKIS(9,9-D
- Page 90 and 91: EFFECTS OF SURFACE CHEMISTRY ON CAD
- Page 92 and 93: EFFECTS OF SURFACE CHEMISTRY ON CAD
- Page 94 and 95: 94 CMDITR Review of Undergraduate R
- Page 96 and 97: SYNTHESIS OF A POLYENE EO CHROMOPHO
- Page 98 and 99: SYNTHESIS OF A POLYENE EO CHROMOPHO
- Page 102 and 103: 102 CMDITR Review of Undergraduate
- Page 106 and 107: 106 CMDITR Review of Undergraduate
- Page 108 and 109: OPTIMIZATION OF SEMICONDUCTOR NANOP
- Page 110 and 111: OPTIMIZATION OF SEMICONDUCTOR NANOP
- Page 112 and 113: CHARACTERIZATION OF THE PHOTODECOMP
- Page 114 and 115: 114 CMDITR Review of Undergraduate
- Page 116 and 117: ELECTROLUMINESCENT PROPERTIES OF OR
- Page 118 and 119: 118 CMDITR Review of Undergraduate
- Page 120 and 121: DETERMINATION OF MOLECULAR ORIENTAT
- Page 122 and 123: DETERMINATION OF MOLECULAR ORIENTAT
- Page 124 and 125: HYDROGEL MATERIALS FOR TWO-PHOTON M
- Page 126 and 127: HYDROGEL MATERIALS FOR TWO-PHOTON M
- Page 128 and 129: THE DESIGN OF A FLUID DELIVERY SYST
- Page 130: THE DESIGN OF A FLUID DELIVERY SYST