Student Project Abstracts 2005 - Pluto - University of Washington
Student Project Abstracts 2005 - Pluto - University of Washington
Student Project Abstracts 2005 - Pluto - University of Washington
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
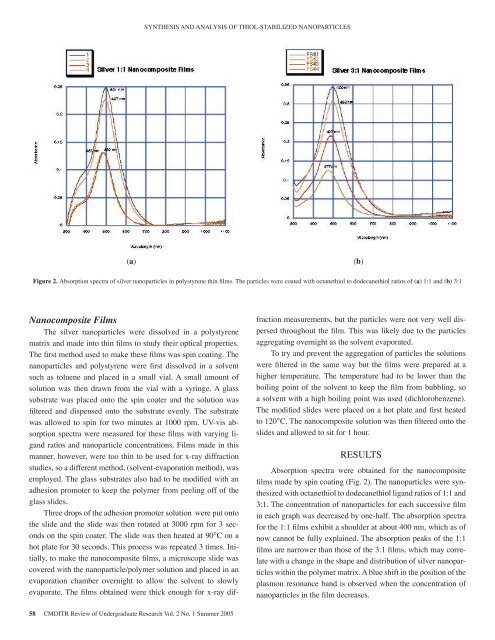
SYNTHESIS AND ANALYSIS OF THIOL-STABILIZED NANOPARTICLES(a)(b)Figure 2. Absorption spectra <strong>of</strong> silver nanoparticles in polystyrene thin films. The particles were coated with octanethiol to dodecanethiol ratios <strong>of</strong> (a) 1:1 and (b) 3:1Nanocomposite FilmsThe silver nanoparticles were dissolved in a polystyrenematrix and made into thin films to study their optical properties.The first method used to make these films was spin coating. Thenanoparticles and polystyrene were first dissolved in a solventsuch as toluene and placed in a small vial. A small amount <strong>of</strong>solution was then drawn from the vial with a syringe. A glasssubstrate was placed onto the spin coater and the solution wasfiltered and dispensed onto the substrate evenly. The substratewas allowed to spin for two minutes at 1000 rpm. UV-vis absorptionspectra were measured for these films with varying ligandratios and nanoparticle concentrations. Films made in thismanner, however, were too thin to be used for x-ray diffractionstudies, so a different method, (solvent-evaporation method), wasemployed. The glass substrates also had to be modified with anadhesion promoter to keep the polymer from peeling <strong>of</strong>f <strong>of</strong> theglass slides.Three drops <strong>of</strong> the adhesion promoter solution were put ontothe slide and the slide was then rotated at 3000 rpm for 3 secondson the spin coater. The slide was then heated at 90°C on ahot plate for 30 seconds. This process was repeated 3 times. Initially,to make the nanocomposite films, a microscope slide wascovered with the nanoparticle/polymer solution and placed in anevaporation chamber overnight to allow the solvent to slowlyevaporate. The films obtained were thick enough for x-ray diffractionmeasurements, but the particles were not very well dispersedthroughout the film. This was likely due to the particlesaggregating overnight as the solvent evaporated.To try and prevent the aggregation <strong>of</strong> particles the solutionswere filtered in the same way but the films were prepared at ahigher temperature. The temperature had to be lower than theboiling point <strong>of</strong> the solvent to keep the film from bubbling, soa solvent with a high boiling point was used (dichlorobenzene).The modified slides were placed on a hot plate and first heatedto 120°C. The nanocomposite solution was then filtered onto theslides and allowed to sit for 1 hour.RESULTSAbsorption spectra were obtained for the nanocompositefilms made by spin coating (Fig. 2). The nanoparticles were synthesizedwith octanethiol to dodecanethiol ligand ratios <strong>of</strong> 1:1 and3:1. The concentration <strong>of</strong> nanoparticles for each successive filmin each graph was decreased by one-half. The absorption spectrafor the 1:1 films exhibit a shoulder at about 400 nm, which as <strong>of</strong>now cannot be fully explained. The absorption peaks <strong>of</strong> the 1:1films are narrower than those <strong>of</strong> the 3:1 films, which may correlatewith a change in the shape and distribution <strong>of</strong> silver nanoparticleswithin the polymer matrix. A blue shift in the position <strong>of</strong> theplasmon resonance band is observed when the concentration <strong>of</strong>nanoparticles in the film decreases.58 CMDITR Review <strong>of</strong> Undergraduate Research Vol. 2 No. 1 Summer <strong>2005</strong>