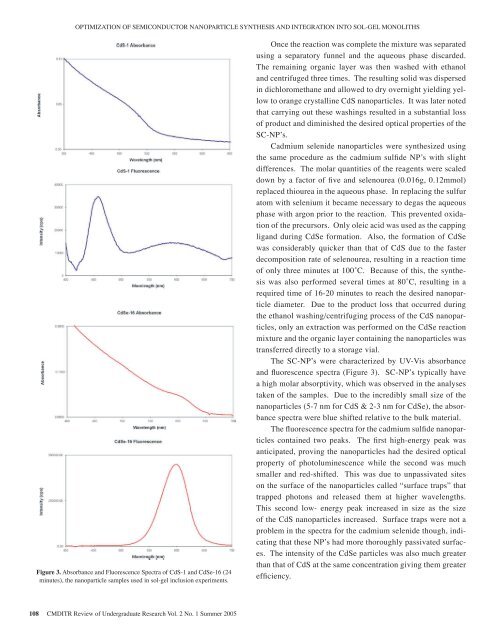
OPTIMIZATION OF SEMICONDUCTOR NANOPARTICLE SYNTHESIS AND INTEGRATION INTO SOL-GEL MONOLITHSFigure 3. Absorbance and Fluorescence Spectra <strong>of</strong> CdS-1 and CdSe-16 (24minutes), the nanoparticle samples used in sol-gel inclusion experiments.Figure 3: Absorbance and Fluorescence Spectra<strong>of</strong> CdS-1 and CdSe-16 (24 minutes), thenanoparticle samples used in sol-gel inclusionexperiments.108 CMDITR Review <strong>of</strong> Undergraduate Research Vol. 2 No. 1 Summer <strong>2005</strong>Once the reaction was complete the mixture was separatedanticipated, proving the nanoparticles hadusing a separatory funnel and the aqueous phase discarded.the desired optical property <strong>of</strong>The remaining organic layer was then washed with ethanolphotoluminescence while the second wasand centrifuged three times. The resulting solid was dispersedmuch smaller and red-shifted. This wasin dichloromethane and allowed to dry overnight yielding yellowtodueorangetocrystallineunpassivatedCdS nanoparticles.sites on theIt wassurfacelater noted<strong>of</strong>thatthecarryingnanoparticlesout these washingscalledresulted“surfacein a substantialtrapsloss”<strong>of</strong> product that trapped and diminished photons the desired and optical released properties them <strong>of</strong> the atSC-NP’s. higher wavelengths. This second low-Cadmium energy peak selenide increased nanoparticles in were size synthesized as the size using <strong>of</strong>the same the procedure CdS nanoparticles as the cadmium increased. sulfide NP’s with Surface slightdifferences. traps were The molar not quantities a problem <strong>of</strong> the in reagents the spectra were scaled fordown the by a cadmium factor <strong>of</strong> five selenide and selenourea though, (0.016g, indicating0.12mmol)replaced that thiourea these in the NP’s aqueous had phase. more In replacing thoroughly the sulfuratom passivated with selenium surfaces. it became necessary The to intensity degas the aqueous <strong>of</strong> thephase CdSe with argon particles prior to was the reaction. also much This prevented greater oxidationthat <strong>of</strong> the <strong>of</strong> precursors. CdS at Only the oleic same acid concentration was used as the capping givingthanligand them during greater CdSe formation. efficiency. Also, the formation <strong>of</strong> CdSewas considerably quicker than that <strong>of</strong> CdS due to the fasterdecomposition rate <strong>of</strong> selenourea, resulting in a reaction timeSol-Gel Formation<strong>of</strong> only three minutes at 100˚C. Because <strong>of</strong> this, the synthesiswas also performed several times at 80˚C, resulting in aSol-gels are formed by the hydrolysis<strong>of</strong> an alkoxide followed by condensationrequired time <strong>of</strong> 16-20 minutes to reach the desired nanoparticlediameter. Due to the product loss that occurred during(Figure 4). Deionized water (0.214 mL,12mmol) and hydrochloric acid (0.56the ethanol washing/centrifuging process <strong>of</strong> the CdS nanoparticles,only an extraction was performed on the CdSe reactionmol) were added to tetramethylorthosilicate (TMOS, 1 mL, 6.7mmol) in amixture and the organic layer containing the nanoparticles wastransferredsmalldirectlyvialtoanda storagestirredvial.for 15 minutes toThehydrolyzeSC-NP’s werethe methylcharacterizedterminatedby UV-Visendsabsorbance<strong>of</strong> theand alkoxide, fluorescence spectra yielding (Figure the 3). precursor SC-NP’s typically solution. havea high Once molar completely absorptivity, which hydrolyzed was observed (evidenced in the analyses bytaken the <strong>of</strong> the evolution samples. Due <strong>of</strong> to the the liquids incredibly to small one size phase) <strong>of</strong> thenanoparticles the precursor (5-7 nm for was CdS & added 2-3 nm for to CdSe), a disposablethe absorbanceacrylate spectra were cuvette blue shifted containing relative to phosphate the bulk material. bufferThe (20mM, fluorescence pH spectra 7, 2 mL) for the and cadmium deionized sulfide nanoparticlesin contained a 1:1 two ratio peaks. with The the first volume high-energy <strong>of</strong> peak solvent waswateranticipated, containing proving nanoparticles.the had the desired opticalproperty <strong>of</strong> photoluminescence while the second was muchsmaller and red-shifted. This was due to unpassivated siteson the surface <strong>of</strong> the nanoparticles called “surface traps” thattrapped photons and released them at higher wavelengths.This Figure second 4: low- Schematic energy peak representation increased in size <strong>of</strong> as hydrolysis the size<strong>of</strong> the CdS and nanoparticles condensation increased. occurring Surface during traps were sol-gel not aproblem in the spectra monolith for the cadmium formation. selenide though, indicatingthat these NP’s had more thoroughly passivated surfaces.The intensityThe first<strong>of</strong> thevariableCdSe particlesoptimizedwas alsowasmuchthegreaterpHthan<strong>of</strong>that <strong>of</strong>theCdS atbufferthe same concentrationsolutiongivingused.them greaterTheefficiency.polymerization (solidifying) rate <strong>of</strong> the solgelincreased in direct proportion with pH.This was initially a problem as the sol-gelwould polymerize quickly, entrapping airbubbles and weakening the matrix. It was
easonable the solvent. amount The <strong>of</strong> desired time while characteristics allowing amajority were a solvent <strong>of</strong> the air the bubbles nanoparticles to escape. would besoluble The second in, water factor miscible, to be and modified one that wasthe would solvent. not quench The the desired luminescence characteristics <strong>of</strong> theSol-Gel werenanoparticles.a Formation solvent the nanoparticlesThis luminescencewould besolublequenching Sol-gels arein,formed effectwaterby was themiscible,hydrolysis determinedand<strong>of</strong> an alkoxideoneto bethatfollowedproblem by condensation with (Figure the 4). first Deionized solvents water (0.214 used, mL,awould12mmol) methanolnotand hydrochloric andquenchethanol.the luminescenceacid (0.56 These μmol) were solvents,<strong>of</strong>added to tetramethylwell orthosilicate as tetrahydr<strong>of</strong>uran Thisasthenanoparticles.(TMOS, 1 mL, 6.7mmol) (THF) luminescencea small vial and andquenchingstirred dimethylformamide effect was 15 minutes to hydrolyze (DMF), determinedthe methyl terminated acted to be as aendsproblem<strong>of</strong> the electron alkoxide, donors, with theyielding the filling firstprecursor in solution. the solvents exciton used,Once completelyhydrolyzed left by (evidenced excited and ethanol.holemethanolby electrons Thesethe evolution in <strong>of</strong> the solvents, liquids SC-NP. asto onewellphase) This the resulted as tetrahydr<strong>of</strong>uranprecursor was in added a lack to a disposable emission (THF)acrylate by cuvette the anddimethylformamidecontaining nanoparticles phosphate and buffer consequently (DMF),(20mM, pH 7, 2 no actedmL) peaks and deionizedin aselectron thewaterspectrum. donors, filling in the exciton holein a 1:1 ratio with the volume <strong>of</strong> solvent containingnanoparticles.left by A excited peak was electrons finally in found the SC-NP. in theThis fluorescence resulted spectra in a lack <strong>of</strong> <strong>of</strong> CdSe-16 emission when by p- thenanoparticles dioxane was used and consequently as the solvent no (Figure peaks 5). inthe The spectrum. peak indicated that this solvent did nothave the A same peak problem was finally as its predecessors. found in thefluorescence p-Dioxane also spectra has intermediate <strong>of</strong> CdSe-16 polarity when so p-dioxane the SC-NP was was used able as the to be solvent dispersed (Figure while 5).The the peak solution indicated remained that water this miscible solvent did giving notthis solvent all the necessary characteristics.have the same problem as its predecessors.p-Dioxane also has intermediate polarity sothe SC-NP was able to be dispersed whilethe solution remained water miscible givingFigure 5: Fluorescence spectrum <strong>of</strong> CdSe-16.24in p-dioxane solvent.this solvent all the necessary characteristics.Figure 4. Schematic representation <strong>of</strong> hydrolysis andcondensation occurring during sol-gel monolith formation.Two additional factors altered from theprinted procedure were temperature and theCdSe-16 in Dioxane0.20Figure 5. Fluorescence spectrum <strong>of</strong> CdSe-16.24 in p-dioxane solvent.Two additional factors altered from the printed procedureWavelength (nmwere temperature and the time <strong>of</strong> nanoparticle addition. Sol-Gelsare very sensitive to temperature and humidity. As time passed,the humidity in the air increased due to seasonal changes (monsoons),affecting the formation <strong>of</strong> the sol-gels. To counteract thiseffect, the temperature at which the precursor was hydrolyzedwas lowered to 10°C. Also, to minimize the effects <strong>of</strong> precursoraddition on the nanoparticles, the SC-NP’s were introduced at thebeginning <strong>of</strong> the hydrolysis step rather than in the buffer solution.By including these small changes in the experimental procedure,transparent sol-gels with known inclusion <strong>of</strong> CdSe nanoparticleswere created (Figure 6).The first variable optimized was the pH <strong>of</strong> the buffer solutionFigure timeused.<strong>of</strong>The 5: nanoparticlepolymerization Fluorescence (solidifying)addition. spectrum rate <strong>of</strong> Sol-Gels<strong>of</strong> CdSe-16.24 the sol-gelareincreasedvery in sensitive direct proportion in p-dioxane to temperature with pH. solvent. This was and initially humidity. a problemas the As sol-gel time would passed, polymerize the quickly, humidity entrapping the air bubbles airand increased weakening Two additional the matrix. due It to factors was determined seasonal altered that changes from a pH <strong>of</strong> the 6.5was printed (monsoons), ideal because procedure the affecting sol-gel were solidified the temperature in formation a reasonable and <strong>of</strong> amount the <strong>of</strong>time sol-gels. while <strong>of</strong> allowing nanoparticle To a majority counteract addition. <strong>of</strong> the air bubbles this Sol-Gels effect, to escape. the arevery temperature Thesensitivesecond factor at to which betemperaturemodified the was precursor theandsolvent.humidity.The was desiredAscharacteristics were a solvent the nanoparticles would behydrolyzed time passed, was lowered the humidity to 10 C. in Also, the to airsoluble in, water miscible, and one that would not quench theincreased minimize the due effects to <strong>of</strong> seasonal precursor addition changesluminescence <strong>of</strong> the nanoparticles. This luminescence quenching(monsoons), the nanoparticles, affecting the formation SC-NP’s <strong>of</strong> were theeffect was determined to be a problem with the first solvents used,sol-gels. introduced To at counteract the beginning this effect, <strong>of</strong> themethanol and ethanol. These solvents, as well as tetrahydr<strong>of</strong>urantemperature hydrolysis step at which rather the than precursor in the buffer was(THF) and dimethylformamide (DMF), acted as electron donors,filling hydrolyzed solution. Byin exciton was includinghole lowered theseleft by excited to electrons 10 small C. changesin Also, the SC-NP. toThis minimizein the experimentalresulted in the a lack effectsprocedure,<strong>of</strong> emission <strong>of</strong> by precursortransparentthe nanoparticles additionsol-gels with known inclusion <strong>of</strong> CdSeand consequentlyonnanoparticlesthe no peaks nanoparticles, inwerethe spectrum.createdthe(FigureSC-NP’s6).wereintroduced A peak was finally at found the in the beginning fluorescence spectra <strong>of</strong> <strong>of</strong> CdSe- the16 hydrolysis when p-dioxane step was used rather as solvent than (Figure in the 5). buffer The peakindicated solution. that this By solvent including did not have these small same problem changes as itspredecessors. in the experimental p-Dioxane also has procedure, intermediate transparentpolarity so theSC-NP sol-gels was able with to be dispersed known while inclusion the solution <strong>of</strong> remained CdSe watermiscible 4 CMDITR giving Review this solvent <strong>of</strong> Undergraduate all the necessary Research characteristics. Vol. 1 No. 1 Summer 2004Figure 6. a) Fluorescence spectrum <strong>of</strong> a CdSe loaded sol-gelmonolith, b) A picture <strong>of</strong> a sol-gel containing nanoparticles.nanoparticles were created (Figure 6).TAYLORNormalized PL1.201.000.800.601.200.40Normalized PL0.200.001.000.80CdSe-16 in Dioxane4 0 0 450 500 5 5 0 6 0 0 6 5 0 7000.600.400.00Wavelength(nm4 0 0 4 5 0 5 00 5 5 0 6 0 0 6 5 0 7 0 0Figure 6: a) Fluorescence spectrum <strong>of</strong> a CdSeloaded sol-gel monolith, b) A picture <strong>of</strong> a sol-gelcontaining nanoparticles.Figure 6: a) Fluorescence spectrum <strong>of</strong> a CdSeloaded sol-gel monolith, b) A picture <strong>of</strong> a sol-gelcontaining nanoparticles.CMDITR Review <strong>of</strong> Undergraduate Research Vol. 2 No. 1 Summer <strong>2005</strong> 109
- Page 2 and 3:
The material is based upon work sup
- Page 4 and 5:
TABLE OF CONTENTSSynthesis of Dendr
- Page 6 and 7:
6 CMDITR Review of Undergraduate Re
- Page 8 and 9:
SYNTHESIS OF DENDRIMER BUILDING BLO
- Page 10 and 11:
throughout the work period. Five su
- Page 12 and 13:
12 CMDITR Review of Undergraduate R
- Page 14 and 15:
BARIUM TITANATE DOPED SOL-GEL FOR E
- Page 16 and 17:
BARIUM TITANATE DOPED SOL-GEL FOR E
- Page 18 and 19:
SYNTHESIS OF NORBORNENE MONOMER OF
- Page 20:
20 CMDITR Review of Undergraduate R
- Page 23 and 24:
using different reaction conditions
- Page 25 and 26:
Synthesis of Nonlinear Optical-Acti
- Page 27 and 28:
quality of the XRD structures wasca
- Page 29 and 30:
Behavioral Properties of Colloidal
- Page 32 and 33:
Transmission electron microscopy ha
- Page 34 and 35:
34 CMDITR Review of Undergraduate R
- Page 36 and 37:
areorient themselves with the elect
- Page 38 and 39:
Fabry-Perot modulators with electro
- Page 40 and 41:
40 CMDITR Review of Undergraduate R
- Page 42 and 43:
QUANTIZED HAMILTON DYNAMICS APPLIED
- Page 44 and 45:
44 CMDITR Review of Undergraduate R
- Page 46 and 47:
INVESTIGATING NEW CLADDING AND CORE
- Page 48 and 49:
Dr. Robert NorwoodChris DeRoseAmir
- Page 50 and 51:
SYNTHESIS OF TPD-BASED COMPOUNDS FO
- Page 52 and 53:
SYNTHESIS OF TPD-BASED COMPOUNDS FO
- Page 54 and 55:
OPTIMIZING HYBRID WAVEGUIDESpropaga
- Page 56 and 57:
At closer spaces the second undesir
- Page 58 and 59: SYNTHESIS AND ANALYSIS OF THIOL-STA
- Page 60 and 61: 60 CMDITR Review of Undergraduate R
- Page 62 and 63: QUINOXALINE-CONTAINING POLYFLUORENE
- Page 64 and 65: QUINOXALINE-CONTAINING POLYFLUORENE
- Page 66 and 67: 66 CMDITR Review of Undergraduate R
- Page 68 and 69: SYNTHESIS OF DENDRON-FUNCTIONALIZED
- Page 70 and 71: 70 CMDITR Review of Undergraduate R
- Page 72 and 73: BUILDING AN OPTICAL OXIMETER TO MEA
- Page 74 and 75: 74 CMDITR Review of Undergraduate R
- Page 76 and 77: 76 CMDITR Review of Undergraduate R
- Page 78 and 79: TOWARD MOLECULAR RESOLUTION C-AFM W
- Page 80 and 81: TOWARD MOLECULAR RESOLUTION C-AFM W
- Page 82 and 83: SYNTHESIS AND CHARACTERIZATION OF E
- Page 84 and 85: My name is Aaron Montgomery and I a
- Page 86 and 87: 1,1-DIPHENYL-2,3,4,5-TETRAKIS(9,9-D
- Page 88 and 89: 1,1-DIPHENYL-2,3,4,5-TETRAKIS(9,9-D
- Page 90 and 91: EFFECTS OF SURFACE CHEMISTRY ON CAD
- Page 92 and 93: EFFECTS OF SURFACE CHEMISTRY ON CAD
- Page 94 and 95: 94 CMDITR Review of Undergraduate R
- Page 96 and 97: SYNTHESIS OF A POLYENE EO CHROMOPHO
- Page 98 and 99: SYNTHESIS OF A POLYENE EO CHROMOPHO
- Page 102 and 103: 102 CMDITR Review of Undergraduate
- Page 104 and 105: CHARACTERIZATION OF THE MOLECULAR P
- Page 106 and 107: 106 CMDITR Review of Undergraduate
- Page 110 and 111: OPTIMIZATION OF SEMICONDUCTOR NANOP
- Page 112 and 113: CHARACTERIZATION OF THE PHOTODECOMP
- Page 114 and 115: 114 CMDITR Review of Undergraduate
- Page 116 and 117: ELECTROLUMINESCENT PROPERTIES OF OR
- Page 118 and 119: 118 CMDITR Review of Undergraduate
- Page 120 and 121: DETERMINATION OF MOLECULAR ORIENTAT
- Page 122 and 123: DETERMINATION OF MOLECULAR ORIENTAT
- Page 124 and 125: HYDROGEL MATERIALS FOR TWO-PHOTON M
- Page 126 and 127: HYDROGEL MATERIALS FOR TWO-PHOTON M
- Page 128 and 129: THE DESIGN OF A FLUID DELIVERY SYST
- Page 130: THE DESIGN OF A FLUID DELIVERY SYST