Student Project Abstracts 2005 - Pluto - University of Washington
Student Project Abstracts 2005 - Pluto - University of Washington
Student Project Abstracts 2005 - Pluto - University of Washington
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
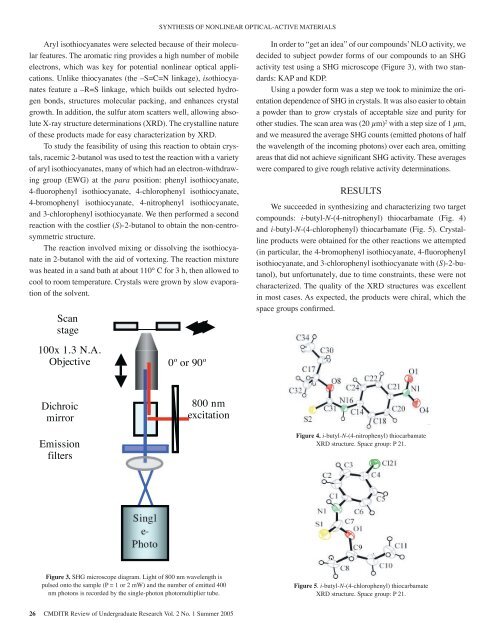
determinations (XRD). The crystalline nature <strong>of</strong> these measured the average SHG counts (emitted phoproducts made for easy characterization by XRD.half the wavelength <strong>of</strong> the incoming photonSYNTHESIS OF NONLINEAR OPTICAL-ACTIVE MATERIALSTo study the feasibility <strong>of</strong> using this reaction to each area, omitting areas that did notobtain Aryl crystals, isothiocyanates racemic were 2-butanol selected because was <strong>of</strong> used their molecularfeatures. with The a aromatic variety ring <strong>of</strong> provides aryl isothiocyanates, a high number <strong>of</strong> mobile many decided to subject compared powder forms to <strong>of</strong> give our compounds rough to an relative SHGto test the In order significant to “get idea” <strong>of</strong> SHG our compounds’ activity. NLO These activity, we averagesreaction<strong>of</strong> electrons, which had which an was electron-withdrawing key for potential nonlinear group optical (EWG) applications.Unlike thiocyanates (the –S=C=N linkage), isothiocyadards:KAP and KDP.at activity test determinations.using a SHG microscope (Figure 3), with two stan-the hydrogen para bonds, position: structures phenyl molecular isothiocyanate, packing, and 4- also easier to obtain a powder than to grow crysnates feature a –R=S linkage, which builds out selected hydrogenbonds, structures molecular packing, and enhances crystal entation dependence <strong>of</strong> SHG in crystals. It was also easier to obtainUsing a powder form was a step we took to minimize the ori-fluorophenyl enhances crystal growth. isothiocyanate, In addition, the 4-chlorophenyl sulfur atom acceptable Results size and purity for other studies. Thisothiocyanate, scatters well, allowinggrowth. In addition, the4-bromophenyl absolutesulfur atom scattersisothiocyanate, X-ray structurewell, allowing absoluteX-ray structure isothiocyanate, (XRD). The crystalline4- areaa powder thanWe wasto growsucceeded (20 µm) 2crystals <strong>of</strong> acceptablein with synthesizing a step sizesize and purityand <strong>of</strong> 1forcharac µm, anitrophenyl determinationsdeterminations (XRD). and The crystalline 3-chlorophenylnature <strong>of</strong> these measurednature other studies. two The scan target the averagearea was compounds: SHG counts(20 µm) 2 with a step i-butyl-N-(4-nitro(emitted phosize <strong>of</strong> 1 µm,isothiocyanate. products<strong>of</strong> these productsmademadefor We easyfor then easycharacterizationcharacterization performed a by second byXRD.XRD. reaction and we measuredhalf thiocarbamate thetheaveragewavelengthSHG (Fig. counts 4) <strong>of</strong>(emitted and the i-butyl-N-(4-chlorophotonsincoming<strong>of</strong> halfphotonswith To To the study costlier the the feasibility feasibility (S)-2-butanol <strong>of</strong> using <strong>of</strong> this using reaction to obtain this to obtain reaction the crys-nontals,racemic crystals, 2-butanol racemic structure. was used 2-butanol to test the reaction was used with a to variety test the areas that did significant obtained not achieve for significant SHG the SHG other activity. reactions These These averages we averages attempcentrosymmetric obtainto the wavelength each thiocarbamate <strong>of</strong> the area, incoming omitting photons) (Fig. over 5). areas each Crystalline area, that omitting did product not areaction <strong>of</strong> aryl isothiocyanates, many which had electron-withdrawinggroup (EWG) at the para position: phenyl isothiocyanate,were compared to give rough relative activity determinations.The reaction with a variety involved <strong>of</strong> mixing aryl isothiocyanates, or dissolving many the compared particular, to the give 4- rough relative aisothiocyanate <strong>of</strong> which had an in electron-withdrawing 2-butanol the aid group <strong>of</strong> vortexing. (EWG) at determinations.bromophenyl4-fluorophenyl isothiocyanate, 4-chlorophenyl RESULTSThe the4-bromophenylreaction para position: mixtureisothiocyanate,was phenyl4-nitrophenylheated isothiocyanate, inisothiocyanate,a sand bath 4- at isothiocyanate, 4-We succeeded in synthesizing and characterizing two targetabout fluorophenyland 3-chlorophenyl 110° C for isothiocyanate. 3 isothiocyanate, h, then We allowed then performed to 4-chlorophenylcool a second to room Results fluorophenylcompounds: i-butyl-N-(4-nitrophenyl) thiocarbamate (Fig. 4)temperature. isothiocyanate,reaction with the costlier Crystals 4-bromophenyl(S)-2-butanol were to obtain grown isothiocyanate,the non-centrosymmetricstructure. <strong>of</strong> the isothiocyanate, solvent. and 3-chlorophenyl two target compounds: i-butyl-N-(4-nitropby slow 4- We succeeded in synthesizing and charactand i-butyl-N-(4-chlorophenyl) thiocarbamate (Fig. 5). Crystallineproducts were obtained for the other reactions we attemptedevaporation nitrophenylctures isothiocyanate. molecular In The order reaction to packing, “get involved We an then mixing idea” and performed <strong>of</strong> dissolving our also compounds’ a the second easier isothiocyanatein 2-butanol with the aid <strong>of</strong> vortexing. The reaction mixturereaction to NLO obtain a powder thiocarbamate than to grow (Fig. crystals 4) and <strong>of</strong> i-butyl-N-(4-chlorop(in particular, the 4-bromophenyl isothiocyanate, 4-fluorophenylth. In activity, with addition, the we costlier the decided sulfur (S)-2-butanol to atom subject powder to acceptable obtain forms the size <strong>of</strong> noncentrosymmetricX-ray to an structure.SHG activity test area using was (20 a SHG µm) 2 tanol), with but a unfortunately, step obtained size <strong>of</strong> for due 1 to the µm, time other and constraints, we reactions these were we not attemptour andisothiocyanate,purity for thiocarbamateandother3-chlorophenylstudies. (Fig. Theisothiocyanate5). scan Crystallinewith (S)-2-bu-productsing was heated in a sand bath at about 110° C for 3 h, then allowed tocompounds absolutecool to room temperature. Crystals were grown by slow evaporation<strong>of</strong> the solvent.. The microscope crystalline The reaction nature characterized. The quality <strong>of</strong> the XRD structures was excellent(Figureinvolved <strong>of</strong> 3), these withmixingtwo standards: measured or dissolvingKAP the average andthe SHG counts particular, (emitted photons the <strong>of</strong> 4-characterization in most cases. As expected, the products were chiral, which isothiocyanattheKDP.isothiocyanate by XRD. in 2-butanol with the half aid the <strong>of</strong> vortexing. wavelength <strong>of</strong> the bromophenyl incoming photons) overspace groups confirmed.ility The <strong>of</strong> using reaction this mixture reaction was to heated each in a sand area, bath omitting at areas isothiocyanate, that did not achieve 4-ScanFigure 5. i-butylc 2-butanol about 110° was C for 3 h, then allowed to cool to roomstageused to test significant SHG activity. fluorophenyl These averages were chlorophenyl)<strong>of</strong> aryl temperature. isothiocyanates, Crystals many were grown compared by to slow give rough Figure relative 4. i-butyl-N-(4- activity thiocarbamate Xn-withdrawing evaporation 100x 1.3 group <strong>of</strong> N.A. the (EWG) solvent. at determinations.nitrophenyl) thiocarbamate structure. Space gphenylIn order Objective isothiocyanate,to “get an idea”4-<strong>of</strong> our 0º compounds’ or 90º NLO XRD structure. Space group: 21.activity, we decided to subject powder forms <strong>of</strong> our P 21.hiocyanate, 4-chlorophenyl Resultscompounds to an SHG activity test using a SHG3-chloromophenyl isothiocyanate, 4- We succeeded in synthesizingmicroscope (Figure 3), with two standards: isothiocyanate and characterizing with (S)-2-butanol), but unfortuDichroic800 nm KAP andyanate, and 3-chlorophenyl two target compounds:KDP.due i-butyl-N-(4-nitrophenyl)to time constraints, these were isothiocyanat noten performed mirror a second reaction thiocarbamate excitation (Fig. 4) and i-butyl-N-(4-chlorophenyl)-2-butanol to Scan obtain the nonture.obtained for the other reactions characterized. Figure we 4. i-butyl-N-(4- attempted The quality (in <strong>of</strong> the XRD structurthiocarbamate (Fig. 5). CrystallineFigure 5. i-butylchlorophenyl)Figure 4. i-butyl-N-(4-nitrophenyl) products were thiocarbamateEmission stageXRD structure. Space group: P 21.filtersthiocarbamate XRved mixing 100x or 1.3 dissolving N.A. the particular, the 4- excellent nitrophenyl) in most thiocarbamate cases. As structure. expected, Space the pgtanol with the Objective aid <strong>of</strong> vortexing. 0º bromophenyl or 90ºwere XRD chiral, structure. which Space the group: space groups 21. confirmed.was heated in a sand bath at isothiocyanate, 4- P When 21. we attempted to form i-butyl-Nthiocarbamate,we were surprised to ob3-chloro, then allowed to cool to room fluorophenylisothiocyanate with (S)-2-butanol), but unfortus were Dichroic grown by slowpreviously unseen productSingl800 nmdue to time constraints, these were notent. mirrorexcitation(according to searches <strong>of</strong>e-idea” <strong>of</strong> our compounds’ NLOthe Cambridge StructuralPhotoo subject Emission powder forms <strong>of</strong> ourUsDatabase and SciFinder)characterized. The quality <strong>of</strong> the XRD structurG activity filters test using a SHGcontaining seven phenylexcellent in most cases. As expected, the pr, with Figure Figuretwo standards: 3. 3. SHG microscope diagram.KAP and diagram. Light <strong>of</strong> 800 nm Light wavelength <strong>of</strong> is 800 nm rings (Fig. 7).pulsed onto the sample (P = 1 or 2 mW) and the number <strong>of</strong> emitted 400Figure were 5. chiral, i-butyl-N-(4-chlorophenyl) which the space groups confirmed.wavelength is pulsed onto the sample (P = 1 or 2 mW) and It is isothiocyanate, thiocarbamateheavily conjugated andnm photons is recorded by the single-photon photomultiplier tube.When XRD structure. we Space attempted group: P 21. to form i-butyl-Nthiocarbamate,Figure 5. i-butyl-N-(4- we were surprised to obtthe number <strong>of</strong> emitted 400 nm photons is recorded by the and also chiral, and therefore predicted tosingle-photon 26 CMDITR Review photomultiplier <strong>of</strong> Undergraduate Research tube. Vol. 2 No. 1 Summer <strong>2005</strong>previouslynonlinear opticalunseenactivity.productHowever, its crystachlorophenyl)ing a powder form was a Singl step we took Figure to minimize 4. i-butyl-N-(4- (accordingnot large enoughto searchesto perform<strong>of</strong>SHG studiesthiocarbamate XRD