Student Project Abstracts 2005 - Pluto - University of Washington
Student Project Abstracts 2005 - Pluto - University of Washington
Student Project Abstracts 2005 - Pluto - University of Washington
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
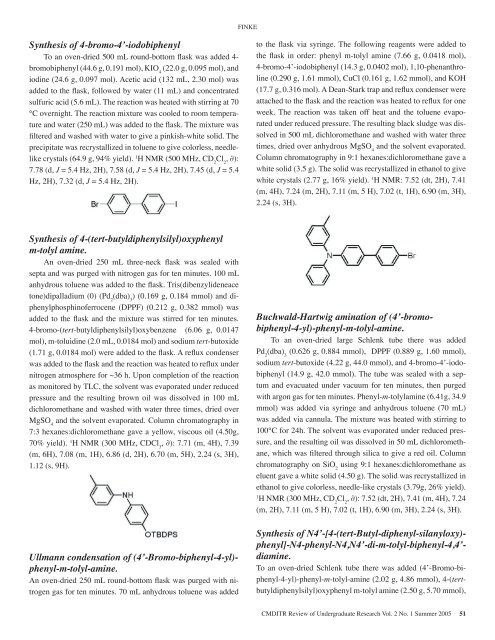
FINKESynthesis <strong>of</strong> 4-bromo-4’-iodobiphenylTo an oven-dried 500 mL round-bottom flask was added 4-bromobiphenyl (44.6 g, 0.191 mol), KIO 4(22.0 g, 0.095 mol), andiodine (24.6 g, 0.097 mol). Acetic acid (132 mL, 2.30 mol) wasadded to the flask, followed by water (11 mL) and concentratedsulfuric acid (5.6 mL). The reaction was heated with stirring at 70°C overnight. The reaction mixture was cooled to room temperatureand water (250 mL) was added to the flask. The mixture wasfiltered and washed with water to give a pinkish-white solid. Theprecipitate was recrystallized in toluene to give colorless, needlelikecrystals (64.9 g, 94% yield). 1 H NMR (500 MHz, CD 2Cl 2, ∂):7.78 (d, J = 5.4 Hz, 2H), 7.58 (d, J = 5.4 Hz, 2H), 7.45 (d, J = 5.4Hz, 2H), 7.32 (d, J = 5.4 Hz, 2H).to the flask via syringe. The following reagents were added tothe flask in order: phenyl m-tolyl amine (7.66 g, 0.0418 mol),4-bromo-4’-iodobiphenyl (14.3 g, 0.0402 mol), 1,10-phenanthroline(0.290 g, 1.61 mmol), CuCl (0.161 g, 1.62 mmol), and KOH(17.7 g, 0.316 mol). A Dean-Stark trap and reflux condenser wereattached to the flask and the reaction was heated to reflux for oneweek. The reaction was taken <strong>of</strong>f heat and the toluene evaporatedunder reduced pressure. The resulting black sludge was dissolvedin 500 mL dichloromethane and washed with water threetimes, dried over anhydrous MgSO 4and the solvent evaporated.Column chromatography in 9:1 hexanes:dichloromethane gave awhite solid (3.5 g). The solid was recrystallized in ethanol to givewhite crystals (2.77 g, 16% yield). 1 H NMR: 7.52 (dt, 2H), 7.41(m, 4H), 7.24 (m, 2H), 7.11 (m, 5 H), 7.02 (t, 1H), 6.90 (m, 3H),2.24 (s, 3H).Synthesis <strong>of</strong> 4-(tert-butyldiphenylsilyl)oxyphenylm-tolyl amine.An oven-dried 250 mL three-neck flask was sealed withsepta and was purged with nitrogen gas for ten minutes. 100 mLanhydrous toluene was added to the flask. Tris(dibenzylideneacetone)dipalladium (0) (Pd 2(dba) 3) (0.169 g, 0.184 mmol) and diphenylphosphin<strong>of</strong>errocene(DPPF) (0.212 g, 0.382 mmol) wasadded to the flask and the mixture was stirred for ten minutes.4-bromo-(tert-butyldiphenylsilyl)oxybenzene (6.06 g, 0.0147mol), m-toluidine (2.0 mL, 0.0184 mol) and sodium tert-butoxide(1.71 g, 0.0184 mol) were added to the flask. A reflux condenserwas added to the flask and the reaction was heated to reflux undernitrogen atmosphere for ~36 h. Upon completion <strong>of</strong> the reactionas monitored by TLC, the solvent was evaporated under reducedpressure and the resulting brown oil was dissolved in 100 mLdichloromethane and washed with water three times, dried overMgSO 4and the solvent evaporated. Column chromatography in7:3 hexanes:dichloromethane gave a yellow, viscous oil (4.50g,70% yield). 1 H NMR (300 MHz, CDCl 3, ∂): 7.71 (m, 4H), 7.39(m, 6H), 7.08 (m, 1H), 6.86 (d, 2H), 6.70 (m, 5H), 2.24 (s, 3H),1.12 (s, 9H).Ullmann condensation <strong>of</strong> (4’-Bromo-biphenyl-4-yl)-phenyl-m-tolyl-amine.An oven-dried 250 mL round-bottom flask was purged with nitrogengas for ten minutes. 70 mL anhydrous toluene was addedBuchwald-Hartwig amination <strong>of</strong> (4’-bromobiphenyl-4-yl)-phenyl-m-tolyl-amine.To an oven-dried large Schlenk tube there was addedPd 2(dba) 3(0.626 g, 0.884 mmol), DPPF (0.889 g, 1.60 mmol),sodium tert-butoxide (4.22 g, 44.0 mmol), and 4-bromo-4’-iodobiphenyl(14.9 g, 42.0 mmol). The tube was sealed with a septumand evacuated under vacuum for ten minutes, then purgedwith argon gas for ten minutes. Phenyl-m-tolylamine (6.41g, 34.9mmol) was added via syringe and anhydrous toluene (70 mL)was added via cannula. The mixture was heated with stirring to100°C for 24h. The solvent was evaporated under reduced pressure,and the resulting oil was dissolved in 50 mL dichloromethane,which was filtered through silica to give a red oil. Columnchromatography on SiO 2using 9:1 hexanes:dichloromethane aseluent gave a white solid (4.50 g). The solid was recrystallized inethanol to give colorless, needle-like crystals (3.79g, 26% yield).1H NMR (300 MHz, CD 2Cl 2, ∂): 7.52 (dt, 2H), 7.41 (m, 4H), 7.24(m, 2H), 7.11 (m, 5 H), 7.02 (t, 1H), 6.90 (m, 3H), 2.24 (s, 3H).Synthesis <strong>of</strong> N4’-[4-(tert-Butyl-diphenyl-silanyloxy)-phenyl]-N4-phenyl-N4,N4’-di-m-tolyl-biphenyl-4,4’-diamine.To an oven-dried Schlenk tube there was added (4’-Bromo-biphenyl-4-yl)-phenyl-m-tolyl-amine(2.02 g, 4.86 mmol), 4-(tertbutyldiphenylsilyl)oxyphenylm-tolyl amine (2.50 g, 5.70 mmol),CMDITR Review <strong>of</strong> Undergraduate Research Vol. 2 No. 1 Summer <strong>2005</strong> 51