Student Project Abstracts 2005 - Pluto - University of Washington
Student Project Abstracts 2005 - Pluto - University of Washington
Student Project Abstracts 2005 - Pluto - University of Washington
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
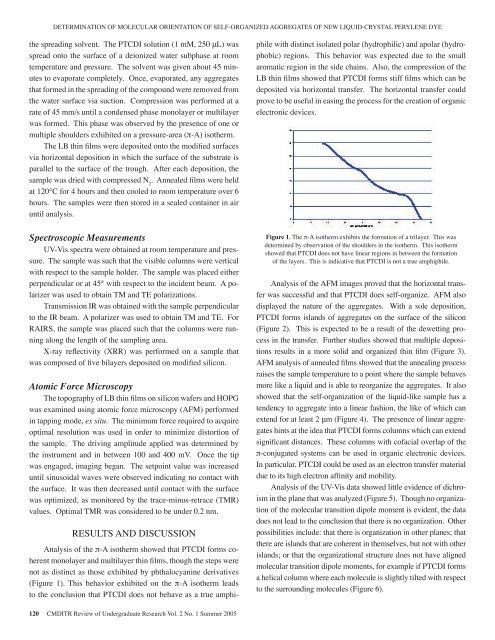
DETERMINATION OF MOLECULAR ORIENTATION OF SELF-ORGANIZED AGGREGATES OF NEW LIQUID-CRYSTAL PERYLENE DYEthe spreading solvent. The PTCDI solution (1 mM, 250 μL) wasspread onto the surface <strong>of</strong> a deionized water subphase at roomtemperature and pressure. The solvent was given about 45 minutesto evaporate completely. Once, evaporated, any aggregatesthat formed in the spreading <strong>of</strong> the compound were removed fromthe water surface via suction. Compression was performed at arate <strong>of</strong> 45 mm/s until a condensed phase monolayer or multilayerwas formed. This phase was observed by the presence <strong>of</strong> one ormultiple shoulders exhibited on a pressure-area (π-A) isotherm.The LB thin films were deposited onto the modified surfacesvia horizontal deposition in which the surface <strong>of</strong> the substrate isparallel to the surface <strong>of</strong> the trough. After each deposition, thesample was dried with compressed N 2. Annealed films were heldat 120°C for 4 hours and then cooled to room temperature over 6hours. The samples were then stored in a sealed container in airuntil analysis.Spectroscopic MeasurementsUV-Vis spectra were obtained at room temperature and pressure.The sample was such that the visible columns were verticalwith respect to the sample holder. The sample was placed eitherperpendicular or at 45° with respect to the incident beam. A polarizerwas used to obtain TM and TE polarizations.Transmission IR was obtained with the sample perpendicularto the IR beam. A polarizer was used to obtain TM and TE. ForRAIRS, the sample was placed such that the columns were runningalong the length <strong>of</strong> the sampling area.X-ray reflectivity (XRR) was performed on a sample thatwas composed <strong>of</strong> five bilayers deposited on modified silicon.Atomic Force MicroscopyThe topography <strong>of</strong> LB thin films on silicon wafers and HOPGwas examined using atomic force microscopy (AFM) performedin tapping mode, ex situ. The minimum force required to acquireoptimal resolution was used in order to minimize distortion <strong>of</strong>the sample. The driving amplitude applied was determined bythe instrument and in between 100 and 400 mV. Once the tipwas engaged, imaging began. The setpoint value was increaseduntil sinusoidal waves were observed indicating no contact withthe surface. It was then decreased until contact with the surfacewas optimized, as monitored by the trace-minus-retrace (TMR)values. Optimal TMR was considered to be under 0.2 nm.RESULTS AND DISCUSSIONAnalysis <strong>of</strong> the π-A isotherm showed that PTCDI forms coherentmonolayer and multilayer thin films, though the steps werenot as distinct as those exhibited by phthalocyanine derivatives(Figure 1). This behavior exhibited on the π-A isotherm leadsto the conclusion that PTCDI does not behave as a true amphiphilewith distinct isolated polar (hydrophilic) and apolar (hydrophobic)regions. This behavior was expected due to the smallaromatic region in the side chains. Also, the compression <strong>of</strong> theLB thin films showed that PTCDI forms stiff films which can bedeposited via horizontal transfer. The horizontal transfer couldprove to be useful in easing the process for the creation <strong>of</strong> organicelectronic devices.Figure 1. The π-A isotherm exhibits the formation <strong>of</strong> a trilayer. This wasdetermined by observation <strong>of</strong> the shoulders in the isotherm. This isothermshowed that PTCDI does not have linear regions in between the formation<strong>of</strong> the layers. This is indicative that PTCDI is not a true amphiphile.Analysis <strong>of</strong> the AFM images proved that the horizontal transferwas successful and that PTCDI does self-organize. AFM alsodisplayed the nature <strong>of</strong> the aggregates. With a sole deposition,PTCDI forms islands <strong>of</strong> aggregates on the surface <strong>of</strong> the silicon(Figure 2). This is expected to be a result <strong>of</strong> the dewetting processin the transfer. Further studies showed that multiple depositionsresults in a more solid and organized thin film (Figure 3).AFM analysis <strong>of</strong> annealed films showed that the annealing processraises the sample temperature to a point where the sample behavesmore like a liquid and is able to reorganize the aggregates. It alsoshowed that the self-organization <strong>of</strong> the liquid-like sample has atendency to aggregate into a linear fashion, the like <strong>of</strong> which canextend for at least 2 μm (Figure 4). The presence <strong>of</strong> linear aggregateshints at the idea that PTCDI forms columns which can extendsignificant distances. These columns with c<strong>of</strong>acial overlap <strong>of</strong> theπ-conjugated systems can be used in organic electronic devices.In particular, PTCDI could be used as an electron transfer materialdue to its high electron affinity and mobility.Analysis <strong>of</strong> the UV-Vis data showed little evidence <strong>of</strong> dichroismin the plane that was analyzed (Figure 5). Though no organization<strong>of</strong> the molecular transition dipole moment is evident, the datadoes not lead to the conclusion that there is no organization. Otherpossibilities include: that there is organization in other planes; thatthere are islands that are coherent in themselves, but not with otherislands; or that the organizational structure does not have alignedmolecular transition dipole moments, for example if PTCDI formsa helical column where each molecule is slightly tilted with respectto the surrounding molecules (Figure 6).120 CMDITR Review <strong>of</strong> Undergraduate Research Vol. 2 No. 1 Summer <strong>2005</strong>