Student Project Abstracts 2005 - Pluto - University of Washington
Student Project Abstracts 2005 - Pluto - University of Washington
Student Project Abstracts 2005 - Pluto - University of Washington
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
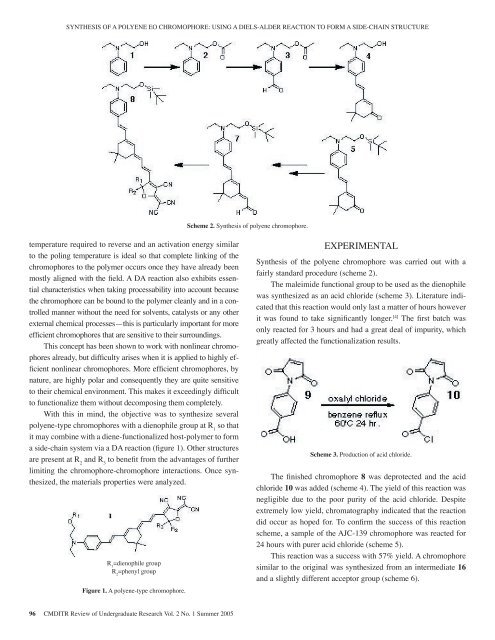
SYNTHESIS OF A POLYENE EO CHROMOPHORE: USING A DIELS-ALDER REACTION TO FORM A SIDE-CHAIN STRUCTUREScheme 2. Synthesis <strong>of</strong> polyene chromophore.temperature required to reverse and an activation energy similarto the poling temperature is ideal so that complete linking <strong>of</strong> thechromophores to the polymer occurs once they have already beenmostly aligned with the field. A DA reaction also exhibits essentialcharacteristics when taking processability into account becausethe chromophore can be bound to the polymer cleanly and in a controlledmanner without the need for solvents, catalysts or any otherexternal chemical processes—this is particularly important for moreefficient chromophores that are sensitive to their surroundings.This concept has been shown to work with nonlinear chromophoresalready, but difficulty arises when it is applied to highly efficientnonlinear chromophores. More efficient chromophores, bynature, are highly polar and consequently they are quite sensitiveto their chemical environment. This makes it exceedingly difficultto functionalize them without decomposing them completely.With this in mind, the objective was to synthesize severalpolyene-type chromophores with a dienophile group at R 1so thatit may combine with a diene-functionalized host-polymer to forma side-chain system via a DA reaction (figure 1). Other structuresare present at R 2and R 3to benefit from the advantages <strong>of</strong> furtherlimiting the chromophore-chromophore interactions. Once synthesized,the materials properties were analyzed.R 1=dienophile groupR 2=phenyl groupFigure 1. A polyene-type chromophore.EXPERIMENTALSynthesis <strong>of</strong> the polyene chromophore was carried out with afairly standard procedure (scheme 2).The maleimide functional group to be used as the dienophilewas synthesized as an acid chloride (scheme 3). Literature indicatedthat this reaction would only last a matter <strong>of</strong> hours howeverit was found to take significantly longer. [4] The first batch wasonly reacted for 3 hours and had a great deal <strong>of</strong> impurity, whichgreatly affected the functionalization results.Scheme 3. Production <strong>of</strong> acid chloride.The finished chromophore 8 was deprotected and the acidchloride 10 was added (scheme 4). The yield <strong>of</strong> this reaction wasnegligible due to the poor purity <strong>of</strong> the acid chloride. Despiteextremely low yield, chromatography indicated that the reactiondid occur as hoped for. To confirm the success <strong>of</strong> this reactionscheme, a sample <strong>of</strong> the AJC-139 chromophore was reacted for24 hours with purer acid chloride (scheme 5).This reaction was a success with 57% yield. A chromophoresimilar to the original was synthesized from an intermediate 16and a slightly different acceptor group (scheme 6).96 CMDITR Review <strong>of</strong> Undergraduate Research Vol. 2 No. 1 Summer <strong>2005</strong>