Student Project Abstracts 2005 - Pluto - University of Washington
Student Project Abstracts 2005 - Pluto - University of Washington
Student Project Abstracts 2005 - Pluto - University of Washington
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
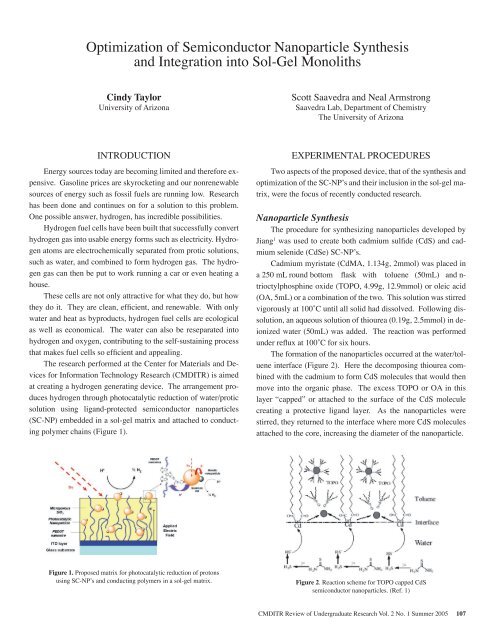
Optimization <strong>of</strong> Semiconductor Nanoparticle Synthesisand Integration into Sol-Gel MonolithsCindy Taylor<strong>University</strong> <strong>of</strong> ArizonaScott Saavedra and Neal ArmstrongSaavedra Lab, Department <strong>of</strong> ChemistryThe <strong>University</strong> <strong>of</strong> ArizonaINTRODUCTIONEnergy sources today are becoming limited and therefore expensive.Gasoline prices are skyrocketing and our nonrenewablesources <strong>of</strong> energy such as fossil fuels are running low. Researchhas been done and continues on for a solution to this problem.One possible answer, hydrogen, has incredible possibilities.Hydrogen fuel cells have been built that successfully converthydrogen gas into usable energy forms such as electricity. Hydrogenatoms are electrochemically separated from protic solutions,such as water, and combined to form hydrogen gas. The hydrogengas can then be put to work running a car or even heating ahouse.These cells are not only attractive for what they do, but howthey do it. They are clean, efficient, and renewable. With onlywater and heat as byproducts, hydrogen fuel cells are ecologicalas well as economical. The water can also be reseparated intohydrogen and oxygen, contributing to the self-sustaining processthat makes fuel cells so efficient and appealing.The research performed at the Center for Materials and Devicesfor Information Technology Research (CMDITR) is aimedat creating a hydrogen generating device. The arrangement produceshydrogen through photocatalytic reduction <strong>of</strong> water/proticsolution using ligand-protected semiconductor nanoparticles(SC-NP) embedded in a sol-gel matrix and attached to conductingpolymer chains (Figure 1).Figure 1. Proposed matrix for photocatalytic reduction <strong>of</strong> protonsusing SC-NP’s and conducting polymers in a sol-gel matrix.onductor Nanoparticlegration into Sol-GelolithsEXPERIMENTAL PROCEDURESTwo aspects <strong>of</strong> the proposed device, that <strong>of</strong> the synthesis andoptimization <strong>of</strong> the SC-NP’s and their inclusion in the sol-gel matrix,were the focus <strong>of</strong> recently conducted research.Nanoparticle SynthesisThe procedure for synthesizing nanoparticles developed byJiang 1 was used to create both cadmium sulfide (CdS) and cadmiumselenide (CdSe) SC-NP’s.Cadmium myristate (CdMA, 1.134g, 2mmol) was placed ina 250 mL round bottom flask with toluene (50mL) and n-trioctylphosphine oxide (TOPO, 4.99g, 12.9mmol) or oleic acid(OA, 5mL) or a combination <strong>of</strong> the two. This solution was stirredvigorously at 100˚C until all solid had dissolved. Following dissolution,an aqueous solution <strong>of</strong> thiourea (0.19g, 2.5mmol) in deionizedwater (50mL) was added. The reaction was performedExperimental Proceduresunder reflux at 100˚C for six hours.Two aspects <strong>of</strong> the proposed device,The that formation <strong>of</strong> the <strong>of</strong> synthesis nanoparticles and optimization occurred the <strong>of</strong> water/tolueneinterface SC-NP’s (Figure and 2). their Here inclusion the decomposing the thiourea sol-gel com-thebined with matrix, the cadmium were the to focus form CdS <strong>of</strong> recently molecules conducted that would thenresearch.move into the organic phase. The excess TOPO or OA in thislayer “capped” or attached to the surface <strong>of</strong> the CdS moleculeNanoparticle Synthesiscreating a protective The procedure ligand layer. for As the synthesizingnanoparticles werestirred, nanoparticles they returned to developed the interface by where Jiang more 1 was CdS used moleculesattached to to create the core, both increasing cadmium the diameter sulfide <strong>of</strong> (CdS) the nanoparticle. andcadmium selenide (CdSe) SC-NP’s.FigureCadmium2. Reactionmyristatescheme for TOPO(CdMA,capped CdS1.134g,semiconductor nanoparticles. (Ref. 1)2mmol) was placed in a 250 mL roundbottom flask with toluene (50mL)and n-trioctylphosphine oxide (TOPO,4.99g, 12.9mmol) or oleic acid (OA, 5mL)or a combination <strong>of</strong> the two. This solutionCMDITR Review <strong>of</strong> Undergraduate Research Vol. 2 No. 1 Summer <strong>2005</strong> 107FiguCdOmixtufunneThewashtimesdichloverncrystnotedresuldiminthe SCsynththedifferreagefivereplareplabecamphasepreveOnlyliganformquickdecomin a100C