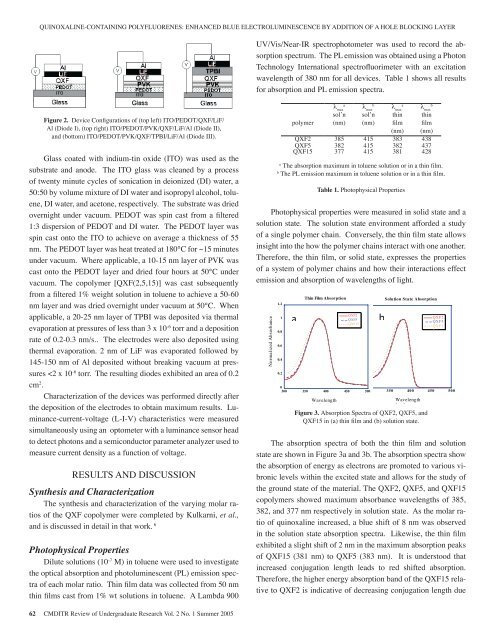
QUINOXALINE-CONTAINING POLYFLUORENES: ENHANCED BLUE ELECTROLUMINESCENCE BY ADDITION OF A HOLE BLOCKING LAYERsol’n sol’n thin thinUV/Vis/Near-IR polymer spectrophotometer (nm) (nm) was used film to record film the absorptionspectrum. The PL emission was obtained (nm) using (nm) a PhotonTechnology QXF2 International 385 spectr<strong>of</strong>luorimeter 415 383 with an 438 excitationwavelength QXF5 <strong>of</strong> 380 nm for 382 all devices. 415 Table 3821 shows 437 all resultsfor absorption QXF15 and PL emission 377 spectra. 415 381 428aThe absorption maximum in toluene solution or inababa thin film. λ maxλ maxλ maxλ max b sol’n sol’n thin thinFigure 2. Device Configurations <strong>of</strong> (top left) ITO/PEDOT/QXF/LiF/The PL emission maximum in toluene solution orin polymer a thin film. (nm) (nm) film filmAl (Diode I), (top right) ITO/PEDOT/PVK/QXF/LiF/Al (Diode II),(nm) (nm)and (bottom) ITO/PEDOT/PVK/QXF/TPBI/LiF/Al (Diode III).QXF2 385 415 383 438Photophysical QXF5 382 properties 415 were 382 measured 437 insolid QXF15 state and 377 a solution 415 state. 381The solution 428aThe state absorption environment maximum afforded in toluene solution a study or <strong>of</strong> in a a thin single film.bThe polymer PL emission chain. maximum Conversely, in toluene solution the thin or in film a thin state film.allows insight into the how the polymer chainsinteract with Table one 1. Photophysical another. Properties Therefore, the thinfilm, or solid state, expresses the properties <strong>of</strong> aPhotophysical system <strong>of</strong> properties polymer were chains measured and in how solid state their and asolution state. interactions The solution effect emission state environment and absorption afforded <strong>of</strong> a studywavelengths <strong>of</strong> light.<strong>of</strong> a single polymer chain. Conversely, the thin film state allowsThe absorption spectra <strong>of</strong> both the thin filminsight intoandthesolutionhow thestatepolymerare shownchainsininteractFigurewith3a andone3b.another.Therefore, The the absorption thin film, or spectra solid show state, expresses the absorption the properties <strong>of</strong><strong>of</strong> a system energy <strong>of</strong> polymer as electrons chains and are how promoted their interactions to various effectemission vibronic and absorption levels <strong>of</strong> within wavelengths the excited <strong>of</strong> light. state andallows for the study <strong>of</strong> the ground state <strong>of</strong> theGlass coated with indium-tin oxide (ITO) was used as thesubstrate and anode. The ITO glass was cleaned by a process<strong>of</strong> twenty minute cycles <strong>of</strong> sonication in deionized (DI) water, a50:50 by volume mixture <strong>of</strong> DI water and isopropyl alcohol, toluene,DI water, and acetone, respectively. The substrate was driedovernight under vacuum. PEDOT was spin cast from a filtered1:3 dispersion <strong>of</strong> PEDOT and DI water. The PEDOT layer wasspin cast onto the ITO to achieve on average a thickness <strong>of</strong> 55nm. The PEDOT layer was heat treated at 180°C for ~15 minutesunder vacuum. Where applicable, a 10-15 nm layer <strong>of</strong> PVK wascast onto the PEDOT layer and dried four hours at 50°C undervacuum. The copolymer [QXF(2,5,15)] was cast subsequentlyfrom a filtered 1% weight solution in toluene to achieve a 50-60nm layer and was dried overnight under vacuum at 50°C. Whenapplicable, a 20-25 nm layer <strong>of</strong> TPBI was deposited via thermalevaporation at pressures <strong>of</strong> less than 3 x 10 -6 torr and a depositionrate <strong>of</strong> 0.2-0.3 nm/s.. The electrodes were also deposited usingthermal evaporation. 2 nm <strong>of</strong> LiF was evaporated followed by145-150 nm <strong>of</strong> Al deposited without breaking vacuum at pressures
max bthinfilm(nm)438437428ion or inution orured insolutionsinglelm stater chainsthe thinties <strong>of</strong> atheirtion <strong>of</strong>It is understood that increased conjugation lengthleads to red shifted absorption. Therefore, thehigher energy absorption band <strong>of</strong> the QXF15relative to QXF2 is indicative <strong>of</strong> decreasingconjugation length due to the increasingto the increasingquinoxalinequinoxalinecontent.content.The quinoxalineThe quinoxalinecontentcontentinterrupts interrupts the conjugation the conjugation along the carbon along backbone the carbon <strong>of</strong> the polymerby introducing backbone <strong>of</strong> kinks the and polymer bends which by introducing disturb the kinks delocalizedelectron and cloud. bends which disturb the delocalized electroncloud.Normalized Intensity1.210.80.60.40.20aThin Film PLE i i400 450 500 550 600Wavelength( )QXF2QXF5QXF15Solution State PL EmissionQXF2QXF5QXF15450 500 550 600Wavelength( )Figure 4. Photoluminescent Emission Emission Spectra <strong>of</strong> Spectra QXF2, <strong>of</strong>in film QXF2, QXF5, QXF5, and QXF15 and QXF15 in (a) thin in film (a) and thin (b) film solution. and (b)and 3b. solution.tion <strong>of</strong>The PL The emission PL emission spectra for spectra both thin for both film and thin solution film statevarious and solution state are shown in Figure 4 withare shown in Figure 4 with maximum emission at 383 nm (QXF2)ate and maximum emission at 383 nm (QXF2) and 438<strong>of</strong> the and 438nmnm (QXF2),(QXF2),respectively.respectively.TheThePLPLemissionemissionisisa snapshotasorption excited state snapshot <strong>of</strong> the excited copolymer state and <strong>of</strong> emission the copolymer is a result and <strong>of</strong> the relaxationemission <strong>of</strong> excited is electrons a result <strong>of</strong> from the the relaxation lowest vibration <strong>of</strong> excited level <strong>of</strong>QXF2the excited electrons state to from the ground the lowest state. vibration The solution level state <strong>of</strong> theQXF5PL emissionspectrum excited (Figure state to 4b) the is red ground shifted state. ~34 nm The from solution the solutionQXF15state PL emission spectrum (Figure 4b) is redstate absorption spectrum, and the thin film PL spectrum (Figureshifted ~34 nm from the solution state4a) is redabsorptionshifted ~50spectrum,from the thinandfilmtheabsorptionthin filmspectrum.PLThethin film spectrum PL emission (Figure spectra 4a) is shows red shifted a ~15 nm ~50 red from shift the relativeto the solution thin film state absorption emission. spectrum. The red The shift thin film the PL spectrasuggests emission increasing spectra conjugation shows length a ~15 as a nm result red <strong>of</strong> shift450 500interchaininteractions relative between to the neighboring solution state polymer emission. and increased The red delocalization<strong>of</strong> electrons that resulted in a lower energyshift in the PL spectra suggests increasingQXF5,emission.bIRVINElectroluminescent PropertiesTable 2 shows the electroluminescent properties <strong>of</strong> Diodes I,II, and III (Figure 2) based on QXF2, QXF5, and QXF15 as theemissive layers. The results for the single layer (Diode I) deviceswill not be shown graphically or in tabular form. The electronicstructures for Diodes I, II, and II are shown in Figure 5. The valuesare <strong>of</strong> the electron affinity (EA) and ionization potential (IP)<strong>of</strong> each material. The electronic structures <strong>of</strong> each diode showthe extent <strong>of</strong> barrier to charge injection between adjacent layersand demonstrate the ease <strong>of</strong> charge mobility through the diode.QXF has a low-lying IP <strong>of</strong> 5.8 eV relative to vacuum level (0 eV).The addition <strong>of</strong> PEDOT as a hole injection layer between the anodeand the emissive layer minimized the barrier to hole injection,but a significant difference <strong>of</strong> 0.6 eV still existed between the IPs<strong>of</strong> PEDOT and QXF. PVK has an ionization potential <strong>of</strong> 5.8 eVthat approaches ohmic contact between the IP <strong>of</strong> QXF and the IP<strong>of</strong> PVK implying that no barrier for the injection <strong>of</strong> holes intothe emissive layer exists. Diode I was fabricated to insure thatDiode II was demonstrating enhanced device performance for allmolar ratios due to the minimization <strong>of</strong> the barrier to charge injection.Kulkarni, et al., completed the cyclic voltammetry <strong>of</strong> thecopolymer and found no significant differences in the ionizationpotential and electron affinity <strong>of</strong> the copolymer relative to PFO. 6However, it was expected that the addition <strong>of</strong> the n-type quinoxalinemoiety would improve the electron transport properties <strong>of</strong>the materials leading to a higher electron affinity. TPBI has alow- lying ionization potential at 6.7 eV that blocks holes andaides in confining charge recombination to the emissive layer.Therefore, the addition <strong>of</strong> the TPBI hole blocking layer could improveperformance and efficiency <strong>of</strong> the devices by minimizingquenching <strong>of</strong> the charges at the interfaces.L max(cd/m 2 )J L max(mA/cm 2 )V a(V)V onλ maxELEQE b%LE c(cd/A)L @ LE(cd/m 2 )CIE 1931(V) (nm)PVK DeviceQXF2 305 170 11 ~5 422 0.81 0.20 235 (0.16, 0.04)QXF5 1180 470 15 ~5 421 0.33 0.25 1180 (0.16, 0.04)QXF15 176 221 11 ~6 421 0.34 0.092 110 (0.16, 0.05)TPBI DeviceQXF2 1620 185 13 ~5 424 1.52 0.91 1530 (0.19, 0.09)QXF5 1510 265 12 ~5 437 1.77 0.71 115 (0.16, 0.05)QXF15 425 137 13 ~6 424 0.81 0.40 165 (0.15, 0.08)PFO 700 170 8 ~4 421 3.2 0.91 108 (0.16, 0.11)aDrive Voltage, b External Quantum Efficiency is defined as the ratio <strong>of</strong>the number <strong>of</strong> photons emitted per number <strong>of</strong> electrons injectedcLuminous Efficiency is a calculation made by dividing the maximumluminance (L max) but the current density at the L max.Table 2. Electroluminescent CharacteristicsCMDITR Review <strong>of</strong> Undergraduate Research Vol. 2 No. 1 Summer <strong>2005</strong> 63
- Page 2 and 3:
The material is based upon work sup
- Page 4 and 5:
TABLE OF CONTENTSSynthesis of Dendr
- Page 6 and 7:
6 CMDITR Review of Undergraduate Re
- Page 8 and 9:
SYNTHESIS OF DENDRIMER BUILDING BLO
- Page 10 and 11:
throughout the work period. Five su
- Page 12 and 13: 12 CMDITR Review of Undergraduate R
- Page 14 and 15: BARIUM TITANATE DOPED SOL-GEL FOR E
- Page 16 and 17: BARIUM TITANATE DOPED SOL-GEL FOR E
- Page 18 and 19: SYNTHESIS OF NORBORNENE MONOMER OF
- Page 20: 20 CMDITR Review of Undergraduate R
- Page 23 and 24: using different reaction conditions
- Page 25 and 26: Synthesis of Nonlinear Optical-Acti
- Page 27 and 28: quality of the XRD structures wasca
- Page 29 and 30: Behavioral Properties of Colloidal
- Page 32 and 33: Transmission electron microscopy ha
- Page 34 and 35: 34 CMDITR Review of Undergraduate R
- Page 36 and 37: areorient themselves with the elect
- Page 38 and 39: Fabry-Perot modulators with electro
- Page 40 and 41: 40 CMDITR Review of Undergraduate R
- Page 42 and 43: QUANTIZED HAMILTON DYNAMICS APPLIED
- Page 44 and 45: 44 CMDITR Review of Undergraduate R
- Page 46 and 47: INVESTIGATING NEW CLADDING AND CORE
- Page 48 and 49: Dr. Robert NorwoodChris DeRoseAmir
- Page 50 and 51: SYNTHESIS OF TPD-BASED COMPOUNDS FO
- Page 52 and 53: SYNTHESIS OF TPD-BASED COMPOUNDS FO
- Page 54 and 55: OPTIMIZING HYBRID WAVEGUIDESpropaga
- Page 56 and 57: At closer spaces the second undesir
- Page 58 and 59: SYNTHESIS AND ANALYSIS OF THIOL-STA
- Page 60 and 61: 60 CMDITR Review of Undergraduate R
- Page 64 and 65: QUINOXALINE-CONTAINING POLYFLUORENE
- Page 66 and 67: 66 CMDITR Review of Undergraduate R
- Page 68 and 69: SYNTHESIS OF DENDRON-FUNCTIONALIZED
- Page 70 and 71: 70 CMDITR Review of Undergraduate R
- Page 72 and 73: BUILDING AN OPTICAL OXIMETER TO MEA
- Page 74 and 75: 74 CMDITR Review of Undergraduate R
- Page 76 and 77: 76 CMDITR Review of Undergraduate R
- Page 78 and 79: TOWARD MOLECULAR RESOLUTION C-AFM W
- Page 80 and 81: TOWARD MOLECULAR RESOLUTION C-AFM W
- Page 82 and 83: SYNTHESIS AND CHARACTERIZATION OF E
- Page 84 and 85: My name is Aaron Montgomery and I a
- Page 86 and 87: 1,1-DIPHENYL-2,3,4,5-TETRAKIS(9,9-D
- Page 88 and 89: 1,1-DIPHENYL-2,3,4,5-TETRAKIS(9,9-D
- Page 90 and 91: EFFECTS OF SURFACE CHEMISTRY ON CAD
- Page 92 and 93: EFFECTS OF SURFACE CHEMISTRY ON CAD
- Page 94 and 95: 94 CMDITR Review of Undergraduate R
- Page 96 and 97: SYNTHESIS OF A POLYENE EO CHROMOPHO
- Page 98 and 99: SYNTHESIS OF A POLYENE EO CHROMOPHO
- Page 102 and 103: 102 CMDITR Review of Undergraduate
- Page 104 and 105: CHARACTERIZATION OF THE MOLECULAR P
- Page 106 and 107: 106 CMDITR Review of Undergraduate
- Page 108 and 109: OPTIMIZATION OF SEMICONDUCTOR NANOP
- Page 110 and 111: OPTIMIZATION OF SEMICONDUCTOR NANOP
- Page 112 and 113:
CHARACTERIZATION OF THE PHOTODECOMP
- Page 114 and 115:
114 CMDITR Review of Undergraduate
- Page 116 and 117:
ELECTROLUMINESCENT PROPERTIES OF OR
- Page 118 and 119:
118 CMDITR Review of Undergraduate
- Page 120 and 121:
DETERMINATION OF MOLECULAR ORIENTAT
- Page 122 and 123:
DETERMINATION OF MOLECULAR ORIENTAT
- Page 124 and 125:
HYDROGEL MATERIALS FOR TWO-PHOTON M
- Page 126 and 127:
HYDROGEL MATERIALS FOR TWO-PHOTON M
- Page 128 and 129:
THE DESIGN OF A FLUID DELIVERY SYST
- Page 130:
THE DESIGN OF A FLUID DELIVERY SYST