School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1.50<br />
HF-HClO 4<br />
carbonate (HNO 3<br />
)<br />
1.25<br />
JUB / reference average<br />
1.00<br />
0.75<br />
0.50<br />
0.25<br />
0.00<br />
Sc Ti Co Ni Rb Sr Y Zr Nb Mo Cs Ba La Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf Ta W Pb Th U<br />
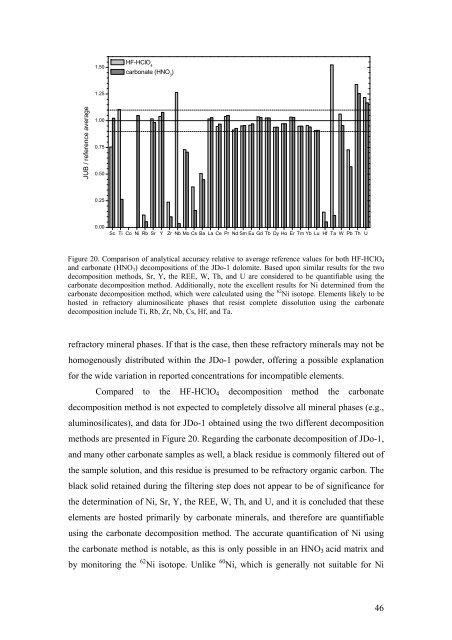
Figure 20. Comparison <strong>of</strong> analytical accuracy relative to average reference values for both HF-HClO 4<br />
<strong>and</strong> carbonate (HNO 3 ) decompositions <strong>of</strong> the JDo-1 dolomite. Based upon similar results for the two<br />
decomposition methods, Sr, Y, the REE, W, Th, <strong>and</strong> U are considered to be quantifiable using the<br />
carbonate decomposition method. Additionally, note the excellent results for Ni determined from the<br />
carbonate decomposition method, which were calculated using the 62 Ni isotope. Elements likely to be<br />
hosted in refractory aluminosilicate phases that resist complete dissolution using the carbonate<br />
decomposition include Ti, Rb, Zr, Nb, Cs, Hf, <strong>and</strong> Ta.<br />
refractory mineral phases. If that is the case, then these refractory minerals may not be<br />
homogenously distributed within the JDo-1 powder, <strong>of</strong>fering a possible explanation<br />
for the wide variation in reported concentrations for incompatible elements.<br />
Compared to the HF-HClO 4 decomposition method the carbonate<br />
decomposition method is not expected to completely dissolve all mineral phases (e.g.,<br />
aluminosilicates), <strong>and</strong> data for JDo-1 obtained using the two different decomposition<br />
methods are presented in Figure 20. Regarding the carbonate decomposition <strong>of</strong> JDo-1,<br />
<strong>and</strong> many other carbonate samples as well, a black residue is commonly filtered out <strong>of</strong><br />
the sample solution, <strong>and</strong> this residue is presumed to be refractory organic carbon. The<br />
black solid retained during the filtering step does not appear to be <strong>of</strong> significance for<br />
the determination <strong>of</strong> Ni, Sr, Y, the REE, W, Th, <strong>and</strong> U, <strong>and</strong> it is concluded that these<br />
elements are hosted primarily by carbonate minerals, <strong>and</strong> therefore are quantifiable<br />
using the carbonate decomposition method. The accurate quantification <strong>of</strong> Ni using<br />
the carbonate method is notable, as this is only possible in an HNO 3 acid matrix <strong>and</strong><br />
by monitoring the 62 Ni isotope. Unlike 60 Ni, which is generally not suitable for Ni<br />
46