School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
10 2 JDo-1 reference values<br />
10 1<br />
IF-G reference values<br />
IQL HCl (n=21)<br />
method blank HCl (n=19)<br />
10 0<br />
10 -1<br />
mg/kg<br />
10 -2<br />
10 -3<br />
10 -4<br />
10 -5<br />
10 -6<br />
10 -7<br />
10 2 Sc Ti Co Ni Rb Sr Y Zr Nb Mo Cs Ba La Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf Ta W Pb Th U<br />
10 1<br />
0.5 M HCl<br />
IQL HNO3 (n=3)<br />
method blank HNO3 (n=3)<br />
10 0<br />
10 -1<br />
mg/kg<br />
10 -2<br />
10 -3<br />
10 -4<br />
10 -5<br />
10 -6<br />
10 -7<br />
0.5 M HNO3<br />
Sc Ti Co Ni Rb Sr Y Zr Nb Mo Cs Ba La Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf Ta W Pb Th U<br />
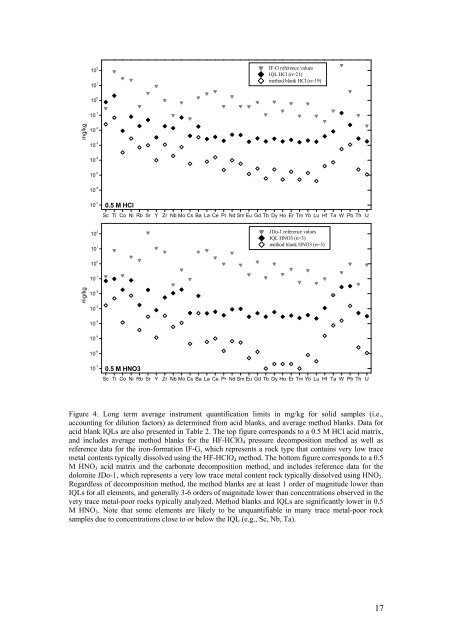
Figure 4. Long term average instrument quantification limits in mg/kg for solid samples (i.e.,<br />
accounting for dilution factors) as determined from acid blanks, <strong>and</strong> average method blanks. Data for<br />
acid blank IQLs are also presented in Table 2. The top figure corresponds to a 0.5 M HCl acid matrix,<br />
<strong>and</strong> includes average method blanks for the HF-HClO 4 pressure decomposition method as well as<br />
reference data for the iron-formation IF-G, which represents a rock type that contains very low trace<br />
metal contents typically dissolved using the HF-HClO 4 method. The bottom figure corresponds to a 0.5<br />
M HNO 3 acid matrix <strong>and</strong> the carbonate decomposition method, <strong>and</strong> includes reference data for the<br />
dolomite JDo-1, which represents a very low trace metal content rock typically dissolved using HNO 3 .<br />
Regardless <strong>of</strong> decomposition method, the method blanks are at least 1 order <strong>of</strong> magnitude lower than<br />
IQLs for all elements, <strong>and</strong> generally 3-6 orders <strong>of</strong> magnitude lower than concentrations observed in the<br />
very trace metal-poor rocks typically analyzed. Method blanks <strong>and</strong> IQLs are significantly lower in 0.5<br />
M HNO 3 . Note that some elements are likely to be unquantifiable in many trace metal-poor rock<br />
samples due to concentrations close to or below the IQL (e.g., Sc, Nb, Ta).<br />
17