School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
30<br />
25<br />
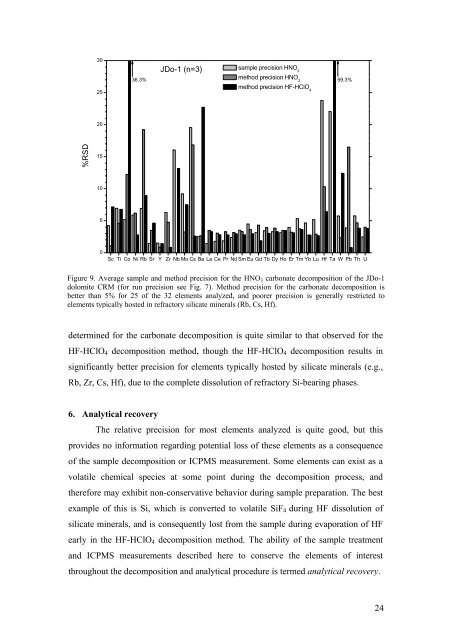
JDo-1 (n=3)<br />
sample precision HNO 3<br />
36.3% method precision HNO 3<br />
59.3%<br />
method precision HF-HClO 4<br />
20<br />
%RSD<br />
15<br />
10<br />
5<br />
0<br />
Sc Ti Co Ni Rb Sr Y Zr Nb Mo Cs Ba La Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf Ta W Pb Th U<br />
Figure 9. Average sample <strong>and</strong> method precision for the HNO 3 carbonate decomposition <strong>of</strong> the JDo-1<br />
dolomite CRM (for run precision see Fig. 7). Method precision for the carbonate decomposition is<br />
better than 5% for 25 <strong>of</strong> the 32 elements analyzed, <strong>and</strong> poorer precision is generally restricted to<br />
elements typically hosted in refractory silicate minerals (Rb, Cs, Hf).<br />
determined for the carbonate decomposition is quite similar to that observed for the<br />
HF-HClO 4 decomposition method, though the HF-HClO 4 decomposition results in<br />
significantly better precision for elements typically hosted by silicate minerals (e.g.,<br />
Rb, Zr, Cs, Hf), due to the complete dissolution <strong>of</strong> refractory Si-bearing phases.<br />
6. Analytical recovery<br />
The relative precision for most elements analyzed is quite good, but this<br />
provides no information regarding potential loss <strong>of</strong> these elements as a consequence<br />
<strong>of</strong> the sample decomposition or ICPMS measurement. Some elements can exist as a<br />
volatile chemical species at some point during the decomposition process, <strong>and</strong><br />
therefore may exhibit non-conservative behavior during sample preparation. The best<br />
example <strong>of</strong> this is Si, which is converted to volatile SiF 4 during HF dissolution <strong>of</strong><br />
silicate minerals, <strong>and</strong> is consequently lost from the sample during evaporation <strong>of</strong> HF<br />
early in the HF-HClO 4 decomposition method. The ability <strong>of</strong> the sample treatment<br />
<strong>and</strong> ICPMS measurements described here to conserve the elements <strong>of</strong> interest<br />
throughout the decomposition <strong>and</strong> analytical procedure is termed analytical recovery.<br />
24