School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
4000<br />
0.40<br />
0.20<br />
3500<br />
0.17 mg/kg<br />
0.18<br />
0.35<br />
interference on 89 Y in sample solution (cps)<br />
3000<br />
2500<br />
2000<br />
1500<br />
1000<br />
500<br />
0.5 M HCl<br />
0.5 M HNO 3<br />
0.13 mg/kg<br />
0.16<br />
0.14<br />
0.12<br />
0.10<br />
0.08<br />
0.06<br />
0.04<br />
0.02<br />
0<br />
0 10 20 30 40 50 60 70 80 90 100<br />
Fe 2<br />
O 3<br />
in sample powder (wt.%)<br />
0.5 M HCl<br />
0.5 M HNO 3<br />
0.30<br />
0.25<br />
0.20<br />
0.15<br />
0.10<br />
0.05<br />
interference on 89 Y in sample powder (mg/kg)<br />
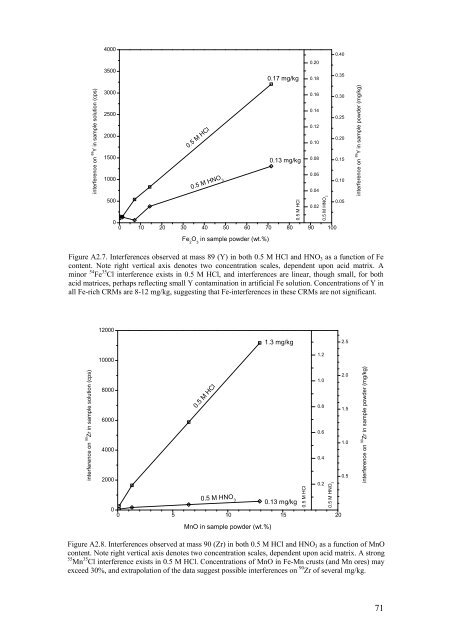
Figure A2.7. Interferences observed at mass 89 (Y) in both 0.5 M HCl <strong>and</strong> HNO 3 as a function <strong>of</strong> Fe<br />
content. Note right vertical axis denotes two concentration scales, dependent upon acid matrix. A<br />
minor 54 Fe 35 Cl interference exists in 0.5 M HCl, <strong>and</strong> interferences are linear, though small, for both<br />
acid matrices, perhaps reflecting small Y contamination in artificial Fe solution. Concentrations <strong>of</strong> Y in<br />
all Fe-rich CRMs are 8-12 mg/kg, suggesting that Fe-interferences in these CRMs are not significant.<br />
12000<br />
1.3 mg/kg<br />
2.5<br />
10000<br />
1.2<br />
interference on 90 Zr in sample solution (cps)<br />
8000<br />
6000<br />
4000<br />
2000<br />
0.5 M HCl<br />
0.5 M HNO 3<br />
0.13 mg/kg<br />
0<br />
0 5 10 15 20<br />
MnO in sample powder (wt.%)<br />
0.5 M HCl<br />
1.0<br />
0.8<br />
0.6<br />
0.4<br />
0.2<br />
0.5 M HNO 3<br />
2.0<br />
1.5<br />
1.0<br />
0.5<br />
interference on 90 Zr in sample powder (mg/kg)<br />
Figure A2.8. Interferences observed at mass 90 (Zr) in both 0.5 M HCl <strong>and</strong> HNO 3 as a function <strong>of</strong> MnO<br />
content. Note right vertical axis denotes two concentration scales, dependent upon acid matrix. A strong<br />
55 Mn 35 Cl interference exists in 0.5 M HCl. Concentrations <strong>of</strong> MnO in Fe-Mn crusts (<strong>and</strong> Mn ores) may<br />
exceed 30%, <strong>and</strong> extrapolation <strong>of</strong> the data suggest possible interferences on 90 Zr <strong>of</strong> several mg/kg.<br />
71