School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
1.35<br />
1.30<br />
1.25<br />
1.20<br />
1.15<br />
HNO 3 carbonate decomp.<br />
HF-HClO 4 decomp.<br />
HF-HClO 4 decomp. pre- June 2006<br />
1.35<br />
1.30<br />
1.25<br />
1.20<br />
1.15<br />
fraction recovered<br />
1.10<br />
1.05<br />
1.00<br />
0.95<br />
0.90<br />
0.85<br />
0.80<br />
0.75<br />
0.30<br />
0.20<br />
0.10<br />
1.10<br />
1.05<br />
1.00<br />
0.95<br />
0.90<br />
0.85<br />
0.80<br />
0.75<br />
0.30<br />
0.20<br />
0.10<br />
Sc Ti Co Ni Rb Sr Y Zr NbMoCs Ba La Ce Pr NdSmEu Gd Tb Dy Ho Er TmYb Lu Hf Ta W Pb Th U<br />
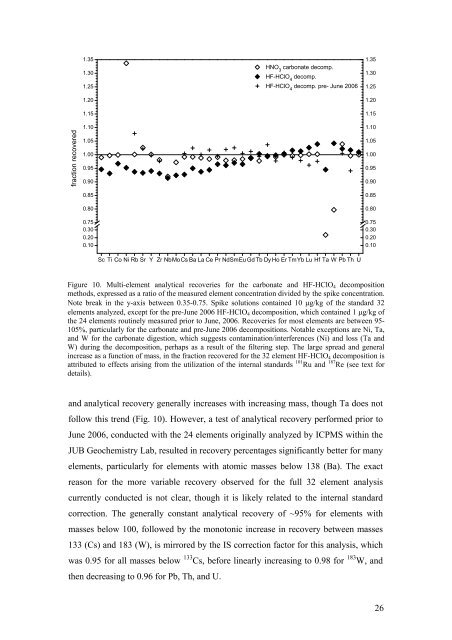
Figure 10. Multi-element analytical recoveries for the carbonate <strong>and</strong> HF-HClO 4 decomposition<br />
methods, expressed as a ratio <strong>of</strong> the measured element concentration divided by the spike concentration.<br />
Note break in the y-axis between 0.35-0.75. Spike solutions contained 10 μg/kg <strong>of</strong> the st<strong>and</strong>ard 32<br />
elements analyzed, except for the pre-June 2006 HF-HClO 4 decomposition, which contained 1 μg/kg <strong>of</strong><br />
the 24 elements routinely measured prior to June, 2006. Recoveries for most elements are between 95-<br />
105%, particularly for the carbonate <strong>and</strong> pre-June 2006 decompositions. Notable exceptions are Ni, Ta,<br />
<strong>and</strong> W for the carbonate digestion, which suggests contamination/interferences (Ni) <strong>and</strong> loss (Ta <strong>and</strong><br />
W) during the decomposition, perhaps as a result <strong>of</strong> the filtering step. The large spread <strong>and</strong> general<br />
increase as a function <strong>of</strong> mass, in the fraction recovered for the 32 element HF-HClO 4 decomposition is<br />
attributed to effects arising from the utilization <strong>of</strong> the internal st<strong>and</strong>ards 101 Ru <strong>and</strong> 187 Re (see text for<br />
details).<br />
<strong>and</strong> analytical recovery generally increases with increasing mass, though Ta does not<br />
follow this trend (Fig. 10). However, a test <strong>of</strong> analytical recovery performed prior to<br />
June 2006, conducted with the 24 elements originally analyzed by ICPMS within the<br />
JUB Geochemistry Lab, resulted in recovery percentages significantly better for many<br />
elements, particularly for elements with atomic masses below 138 (Ba). The exact<br />
reason for the more variable recovery observed for the full 32 element analysis<br />
currently conducted is not clear, though it is likely related to the internal st<strong>and</strong>ard<br />
correction. The generally constant analytical recovery <strong>of</strong> ~95% for elements with<br />
masses below 100, followed by the monotonic increase in recovery between masses<br />
133 (Cs) <strong>and</strong> 183 (W), is mirrored by the IS correction factor for this analysis, which<br />
was 0.95 for all masses below 133 Cs, before linearly increasing to 0.98 for 183 W, <strong>and</strong><br />
then decreasing to 0.96 for Pb, Th, <strong>and</strong> U.<br />
26