School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
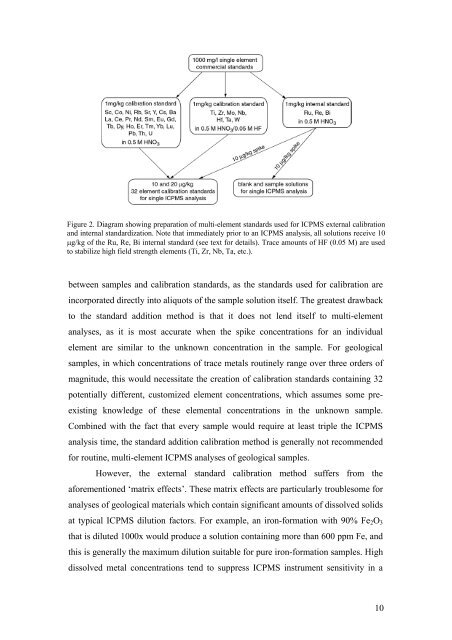
Figure 2. Diagram showing preparation <strong>of</strong> multi-element st<strong>and</strong>ards used for ICPMS external calibration<br />
<strong>and</strong> internal st<strong>and</strong>ardization. Note that immediately prior to an ICPMS analysis, all solutions receive 10<br />
μg/kg <strong>of</strong> the Ru, Re, Bi internal st<strong>and</strong>ard (see text for details). Trace amounts <strong>of</strong> HF (0.05 M) are used<br />
to stabilize high field strength elements (Ti, Zr, Nb, Ta, etc.).<br />
between samples <strong>and</strong> calibration st<strong>and</strong>ards, as the st<strong>and</strong>ards used for calibration are<br />
incorporated directly into aliquots <strong>of</strong> the sample solution itself. The greatest drawback<br />
to the st<strong>and</strong>ard addition method is that it does not lend itself to multi-element<br />
analyses, as it is most accurate when the spike concentrations for an individual<br />
element are similar to the unknown concentration in the sample. For geological<br />
samples, in which concentrations <strong>of</strong> trace metals routinely range over three orders <strong>of</strong><br />
magnitude, this would necessitate the creation <strong>of</strong> calibration st<strong>and</strong>ards containing 32<br />
potentially different, customized element concentrations, which assumes some preexisting<br />
knowledge <strong>of</strong> these elemental concentrations in the unknown sample.<br />
Combined with the fact that every sample would require at least triple the ICPMS<br />
analysis time, the st<strong>and</strong>ard addition calibration method is generally not recommended<br />
for routine, multi-element ICPMS analyses <strong>of</strong> geological samples.<br />
However, the external st<strong>and</strong>ard calibration method suffers from the<br />
aforementioned ‘matrix effects’. These matrix effects are particularly troublesome for<br />
analyses <strong>of</strong> geological materials which contain significant amounts <strong>of</strong> dissolved solids<br />
at typical ICPMS dilution factors. For example, an iron-formation with 90% Fe 2 O 3<br />
that is diluted 1000x would produce a solution containing more than 600 ppm Fe, <strong>and</strong><br />
this is generally the maximum dilution suitable for pure iron-formation samples. High<br />
dissolved metal concentrations tend to suppress ICPMS instrument sensitivity in a<br />
10