School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
100<br />
MCO + 3 + M(CO 3 )- 2<br />
rare earth element<br />
% total<br />
80<br />
60<br />
40<br />
20<br />
MCO + 3<br />
M(CO 3<br />
) - 2<br />
M 3+<br />
0<br />
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu<br />
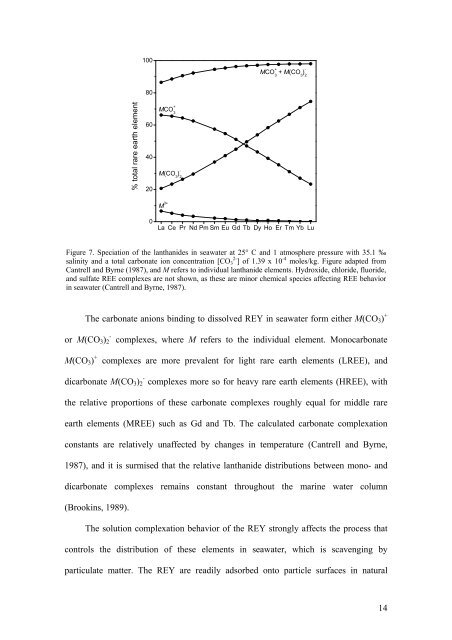
Figure 7. Speciation <strong>of</strong> the lanthanides in seawater at 25° C <strong>and</strong> 1 atmosphere pressure with 35.1 ‰<br />
salinity <strong>and</strong> a total carbonate ion concentration [CO 3 2- ] <strong>of</strong> 1.39 x 10 -4 moles/kg. Figure adapted from<br />
Cantrell <strong>and</strong> Byrne (1987), <strong>and</strong> M refers to individual lanthanide elements. Hydroxide, chloride, fluoride,<br />
<strong>and</strong> sulfate REE complexes are not shown, as these are minor chemical species affecting REE behavior<br />
in seawater (Cantrell <strong>and</strong> Byrne, 1987).<br />
The carbonate anions binding to dissolved REY in seawater form either M(CO 3 ) +<br />
or M(CO 3 ) 2<br />
-<br />
complexes, where M refers to the individual element. Monocarbonate<br />
M(CO 3 ) +<br />
complexes are more prevalent for light rare earth elements (LREE), <strong>and</strong><br />
dicarbonate M(CO 3 ) - 2 complexes more so for heavy rare earth elements (HREE), with<br />
the relative proportions <strong>of</strong> these carbonate complexes roughly equal for middle rare<br />
earth elements (MREE) such as Gd <strong>and</strong> Tb. The calculated carbonate complexation<br />
constants are relatively unaffected by changes in temperature (Cantrell <strong>and</strong> Byrne,<br />
1987), <strong>and</strong> it is surmised that the relative lanthanide distributions between mono- <strong>and</strong><br />
dicarbonate complexes remains constant throughout the marine water column<br />
(Brookins, 1989).<br />
The solution complexation behavior <strong>of</strong> the REY strongly affects the process that<br />
controls the distribution <strong>of</strong> these elements in seawater, which is scavenging by<br />
particulate matter. The REY are readily adsorbed onto particle surfaces in natural<br />
14