School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
16000<br />
0.5 M HCl matrix<br />
a<br />
1.6<br />
14000<br />
1.4<br />
interference on 59 Co in sample solution (cps)<br />
12000<br />
10000<br />
8000<br />
6000<br />
4000<br />
2000<br />
MgCl +<br />
CaO(H) +<br />
MgCl + interference in JDo-1<br />
CaO(H) + interference in JDo-1<br />
1.2<br />
1.0<br />
0.8<br />
0.6<br />
0.4<br />
0.2<br />
interference on 59 Co in sample powder (mg/kg)<br />
0<br />
0.0<br />
0 10 20 30 40 50 60 70 80 90 100<br />
MgO, CaO in sample powder (wt.%)<br />
16000<br />
0.5 M HNO 3<br />
matrix<br />
b<br />
1.6<br />
14000<br />
1.4<br />
interference on 59 Co in sample solution (cps)<br />
12000<br />
10000<br />
8000<br />
6000<br />
4000<br />
2000<br />
CaO(H) +<br />
CaO(H) + interference in JDo-1<br />
1.2<br />
1.0<br />
0.8<br />
0.6<br />
0.4<br />
0.2<br />
interference on 59 Co in sample powder (mg/kg)<br />
0<br />
MgCl +<br />
0.0<br />
0 10 20 30 40 50 60 70 80 90 100<br />
MgO, CaO in sample powder (wt.%)<br />
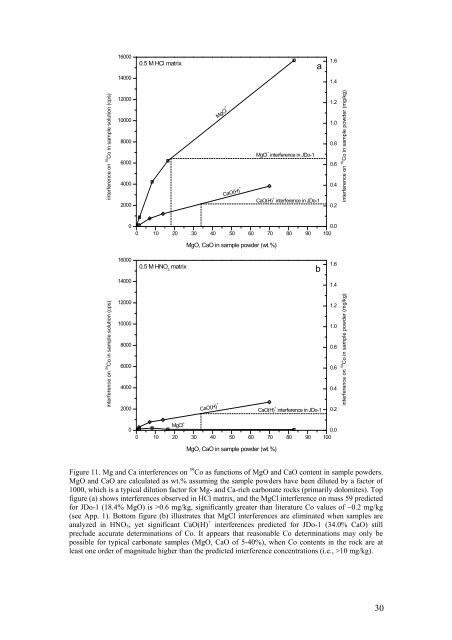
Figure 11. Mg <strong>and</strong> Ca interferences on 59 Co as functions <strong>of</strong> MgO <strong>and</strong> CaO content in sample powders.<br />
MgO <strong>and</strong> CaO are calculated as wt.% assuming the sample powders have been diluted by a factor <strong>of</strong><br />
1000, which is a typical dilution factor for Mg- <strong>and</strong> Ca-rich carbonate rocks (primarily dolomites). Top<br />
figure (a) shows interferences observed in HCl matrix, <strong>and</strong> the MgCl interference on mass 59 predicted<br />
for JDo-1 (18.4% MgO) is >0.6 mg/kg, significantly greater than literature Co values <strong>of</strong> ~0.2 mg/kg<br />
(see App. 1). Bottom figure (b) illustrates that MgCl interferences are eliminated when samples are<br />
analyzed in HNO 3 , yet significant CaO(H) + interferences predicted for JDo-1 (34.0% CaO) still<br />
preclude accurate determinations <strong>of</strong> Co. It appears that reasonable Co determinations may only be<br />
possible for typical carbonate samples (MgO, CaO <strong>of</strong> 5-40%), when Co contents in the rock are at<br />
least one order <strong>of</strong> magnitude higher than the predicted interference concentrations (i.e., >10 mg/kg).<br />
30