Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Study details and Participant details Intervention details Results Conclusion and<br />
design comments<br />
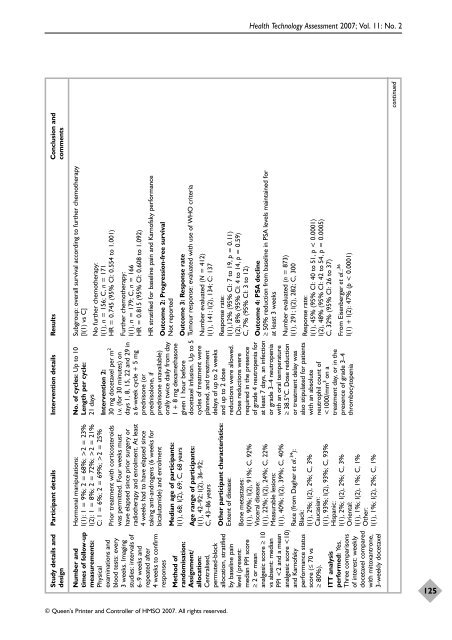
Subgroup: overall survival acc<strong>or</strong>ding to fur<strong>the</strong>r chemo<strong>the</strong>rapy<br />
[I(1) vs C]<br />
No fur<strong>the</strong>r chemo<strong>the</strong>rapy:<br />
I(1), n = 156; C, n = 171<br />
HR = 0.745 (95% CI: 0.554 to 1.001)<br />
H<strong>or</strong>monal manipulations:<br />
I(1): 1 = 9%; 2 = 68%; >2 = 23%<br />
I(2): 1 = 8%; 2 = 72%; >2 = 21%<br />
C: 1 = 6%; 2 = 69%; >2 = 25%<br />
Fur<strong>the</strong>r chemo<strong>the</strong>rapy:<br />
I(1), n = 179; C, n = 166<br />
HR = 0.815 (95% CI: 0.608 to 1.092)<br />
HR stratified f<strong>or</strong> baseline pain and Karn<strong>of</strong>sky perf<strong>or</strong>mance<br />
Pri<strong>or</strong> <strong>treatment</strong> <strong>with</strong> c<strong>or</strong>ticosteroids<br />
was permitted. Four weeks must<br />
have elapsed since pri<strong>or</strong> surgery <strong>or</strong><br />
radio<strong>the</strong>rapy and enrolment. At least<br />
4 weeks had to have elapsed since<br />
taking anti-androgens (6 weeks f<strong>or</strong><br />
bicalutamide) and enrolment<br />
Outcome 2: Progression-free survival<br />
Not rep<strong>or</strong>ted<br />
Number and<br />
times <strong>of</strong> follow-up<br />
measurements:<br />
Physical<br />
examinations and<br />
blood tests: every<br />
3 weeks. Imaging<br />
studies: intervals <strong>of</strong><br />
6–9 weeks and<br />
repeated after<br />
4 weeks to confirm<br />
responses<br />
Median age <strong>of</strong> participants:<br />
I(1), 68; I(2), 69; C, 68 years<br />
Outcome 3: Response rate<br />
Tumour response: evaluated <strong>with</strong> use <strong>of</strong> WHO criteria<br />
Number evaluated (N = 412)<br />
I(1), 141; I(2), 134; C: 137<br />
Age range <strong>of</strong> participants:<br />
I(1), 42–92; I(2), 36–92;<br />
C, 43–86 years<br />
O<strong>the</strong>r participant characteristics:<br />
Extent <strong>of</strong> disease:<br />
© Queen’s Printer and Controller <strong>of</strong> HMSO 2007. All rights reserved.<br />
Response rate:<br />
I(1),12% (95% CI: 7 to 19, p = 0.11)<br />
I(2), 8% (95% CI: 4 to 14, p = 0.59)<br />
C, 7% (95% CI: 3 to 12)<br />
Outcome 4: PSA decline<br />
≥ 50% reduction from baseline in PSA levels maintained f<strong>or</strong><br />
at least 3 weeks<br />
Method <strong>of</strong><br />
randomisation:<br />
Assignment/<br />
allocation:<br />
Centralised,<br />
permuted-block<br />
allocation, stratified<br />
by baseline pain<br />
level (present:<br />
median PPI sc<strong>or</strong>e<br />
≥ 2 <strong>or</strong> mean<br />
analgesic sc<strong>or</strong>e ≥ 10<br />
vs absent: median<br />
PPI