Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
34<br />
Results<br />
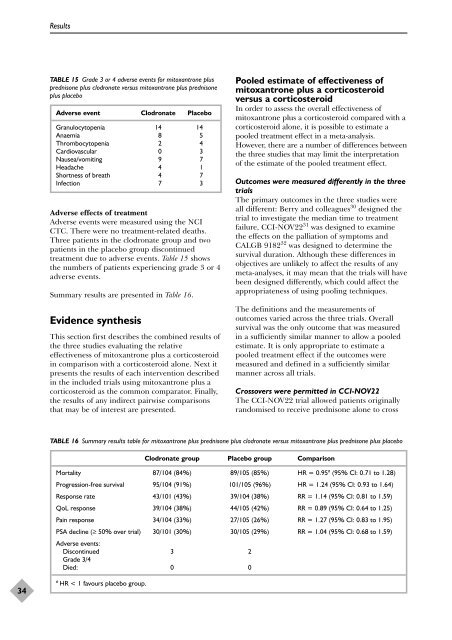
TABLE 15 Grade 3 <strong>or</strong> 4 adverse events f<strong>or</strong> mitoxantrone plus<br />
<strong>prednisone</strong> plus clodronate versus mitoxantrone plus <strong>prednisone</strong><br />
plus placebo<br />
Adverse event Clodronate Placebo<br />
Granulocytopenia 14 14<br />
Anaemia 8 5<br />
Thrombocytopenia 2 4<br />
Cardiovascular 0 3<br />
Nausea/vomiting 9 7<br />
Headache 4 1<br />
Sh<strong>or</strong>tness <strong>of</strong> breath 4 7<br />
Infection 7 3<br />
Adverse effects <strong>of</strong> <strong>treatment</strong><br />
Adverse events were measured using <strong>the</strong> NCI<br />
CTC. There were no <strong>treatment</strong>-related deaths.<br />
Three patients in <strong>the</strong> clodronate group and two<br />
patients in <strong>the</strong> placebo group discontinued<br />
<strong>treatment</strong> due to adverse events. Table 15 shows<br />
<strong>the</strong> numbers <strong>of</strong> patients experiencing grade 3 <strong>or</strong> 4<br />
adverse events.<br />
Summary results are presented in Table 16.<br />
Evidence syn<strong>the</strong>sis<br />
This section first describes <strong>the</strong> combined results <strong>of</strong><br />
<strong>the</strong> three studies evaluating <strong>the</strong> relative<br />
effectiveness <strong>of</strong> mitoxantrone plus a c<strong>or</strong>ticosteroid<br />
in comparison <strong>with</strong> a c<strong>or</strong>ticosteroid alone. Next it<br />
presents <strong>the</strong> results <strong>of</strong> each intervention described<br />
in <strong>the</strong> included trials using mitoxantrone plus a<br />
c<strong>or</strong>ticosteroid as <strong>the</strong> common comparat<strong>or</strong>. Finally,<br />
<strong>the</strong> results <strong>of</strong> any indirect pairwise comparisons<br />
that may be <strong>of</strong> interest are presented.<br />
Pooled estimate <strong>of</strong> effectiveness <strong>of</strong><br />
mitoxantrone plus a c<strong>or</strong>ticosteroid<br />
versus a c<strong>or</strong>ticosteroid<br />
In <strong>or</strong>der to assess <strong>the</strong> overall effectiveness <strong>of</strong><br />
mitoxantrone plus a c<strong>or</strong>ticosteroid compared <strong>with</strong> a<br />
c<strong>or</strong>ticosteroid alone, it is possible to estimate a<br />
pooled <strong>treatment</strong> effect in a meta-analysis.<br />
However, <strong>the</strong>re are a number <strong>of</strong> differences between<br />
<strong>the</strong> three studies that may limit <strong>the</strong> interpretation<br />
<strong>of</strong> <strong>the</strong> estimate <strong>of</strong> <strong>the</strong> pooled <strong>treatment</strong> effect.<br />
Outcomes were measured differently in <strong>the</strong> three<br />
trials<br />
The primary outcomes in <strong>the</strong> three studies were<br />
all different: Berry and colleagues 30 designed <strong>the</strong><br />
trial to investigate <strong>the</strong> median time to <strong>treatment</strong><br />
failure, CCI-NOV22 31 was designed to examine<br />
<strong>the</strong> effects on <strong>the</strong> palliation <strong>of</strong> symptoms and<br />
CALGB 9182 32 was designed to determine <strong>the</strong><br />
survival duration. Although <strong>the</strong>se differences in<br />
objectives are unlikely to affect <strong>the</strong> results <strong>of</strong> any<br />
meta-analyses, it may mean that <strong>the</strong> trials will have<br />
been designed differently, which could affect <strong>the</strong><br />
appropriateness <strong>of</strong> using pooling techniques.<br />
The definitions and <strong>the</strong> measurements <strong>of</strong><br />
outcomes varied across <strong>the</strong> three trials. Overall<br />
survival was <strong>the</strong> only outcome that was measured<br />
in a sufficiently similar manner to allow a pooled<br />
estimate. It is only appropriate to estimate a<br />
pooled <strong>treatment</strong> effect if <strong>the</strong> outcomes were<br />
measured and defined in a sufficiently similar<br />
manner across all trials.<br />
Crossovers were permitted in CCI-NOV22<br />
The CCI-NOV22 trial allowed patients <strong>or</strong>iginally<br />
randomised to receive <strong>prednisone</strong> alone to cross<br />
TABLE 16 Summary results table f<strong>or</strong> mitoxantrone plus <strong>prednisone</strong> plus clodronate versus mitoxantrone plus <strong>prednisone</strong> plus placebo<br />
Clodronate group Placebo group Comparison<br />
M<strong>or</strong>tality 87/104 (84%) 89/105 (85%) HR = 0.95a (95% CI: 0.71 to 1.28)<br />
Progression-free survival 95/104 (91%) 101/105 (96%) HR = 1.24 (95% CI: 0.93 to 1.64)<br />
Response rate 43/101 (43%) 39/104 (38%) RR = 1.14 (95% CI: 0.81 to 1.59)<br />
QoL response 39/104 (38%) 44/105 (42%) RR = 0.89 (95% CI: 0.64 to 1.25)<br />
Pain response 34/104 (33%) 27/105 (26%) RR = 1.27 (95% CI: 0.83 to 1.95)<br />
PSA decline (≥ 50% over trial)<br />
Adverse events:<br />
30/101 (30%) 30/105 (29%) RR = 1.04 (95% CI: 0.68 to 1.59)<br />
Discontinued<br />
Grade 3/4<br />
3 2<br />
Died: 0 0<br />
a HR < 1 favours placebo group.