Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
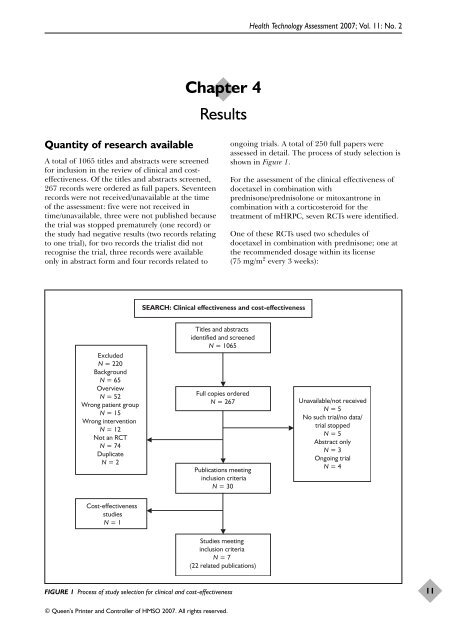
Quantity <strong>of</strong> research available<br />
A total <strong>of</strong> 1065 titles and abstracts were screened<br />
f<strong>or</strong> inclusion in <strong>the</strong> review <strong>of</strong> clinical and costeffectiveness.<br />
Of <strong>the</strong> titles and abstracts screened,<br />
267 rec<strong>or</strong>ds were <strong>or</strong>dered as full papers. Seventeen<br />
rec<strong>or</strong>ds were not received/unavailable at <strong>the</strong> time<br />
<strong>of</strong> <strong>the</strong> assessment: five were not received in<br />
time/unavailable, three were not published because<br />
<strong>the</strong> trial was stopped prematurely (one rec<strong>or</strong>d) <strong>or</strong><br />
<strong>the</strong> study had negative results (two rec<strong>or</strong>ds relating<br />
to one trial), f<strong>or</strong> two rec<strong>or</strong>ds <strong>the</strong> trialist did not<br />
recognise <strong>the</strong> trial, three rec<strong>or</strong>ds were available<br />
only in abstract f<strong>or</strong>m and four rec<strong>or</strong>ds related to<br />
Excluded<br />
N = 220<br />
Background<br />
N = 65<br />
Overview<br />
N = 52<br />
Wrong patient group<br />
N = 15<br />
Wrong intervention<br />
N = 12<br />
Not an RCT<br />
N = 74<br />
Duplicate<br />
N = 2<br />
Cost-effectiveness<br />
studies<br />
N = 1<br />
Chapter 4<br />
Results<br />
SEARCH: Clinical effectiveness and cost-effectiveness<br />
Titles and abstracts<br />
identified and screened<br />
N = 1065<br />
Full copies <strong>or</strong>dered<br />
N = 267<br />
Publications meeting<br />
inclusion criteria<br />
N = 30<br />
Studies meeting<br />
inclusion criteria<br />
N = 7<br />
(22 related publications)<br />
FIGURE 1 Process <strong>of</strong> study selection f<strong>or</strong> clinical and cost-effectiveness<br />
© Queen’s Printer and Controller <strong>of</strong> HMSO 2007. All rights reserved.<br />
Health Technology Assessment 2007; Vol. 11: No. 2<br />
ongoing trials. A total <strong>of</strong> 250 full papers were<br />
assessed in detail. The process <strong>of</strong> study selection is<br />
shown in Figure 1.<br />
F<strong>or</strong> <strong>the</strong> assessment <strong>of</strong> <strong>the</strong> clinical effectiveness <strong>of</strong><br />
docetaxel in combination <strong>with</strong><br />
<strong>prednisone</strong>/<strong>prednisolone</strong> <strong>or</strong> mitoxantrone in<br />
combination <strong>with</strong> a c<strong>or</strong>ticosteroid f<strong>or</strong> <strong>the</strong><br />
<strong>treatment</strong> <strong>of</strong> mHRPC, seven RCTs were identified.<br />
One <strong>of</strong> <strong>the</strong>se RCTs used two schedules <strong>of</strong><br />
docetaxel in combination <strong>with</strong> <strong>prednisone</strong>; one at<br />
<strong>the</strong> recommended dosage <strong>with</strong>in its license<br />
(75 mg/m 2 every 3 weeks):<br />
Unavailable/not received<br />
N = 5<br />
No such trial/no data/<br />
trial stopped<br />
N = 5<br />
Abstract only<br />
N = 3<br />
Ongoing trial<br />
N = 4<br />
11