Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
22<br />
Results<br />
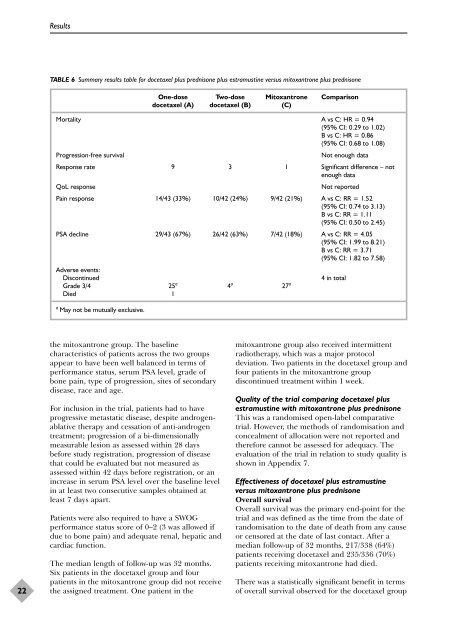
TABLE 6 Summary results table f<strong>or</strong> docetaxel plus <strong>prednisone</strong> plus estramustine versus mitoxantrone plus <strong>prednisone</strong><br />
<strong>the</strong> mitoxantrone group. The baseline<br />
characteristics <strong>of</strong> patients across <strong>the</strong> two groups<br />
appear to have been well balanced in terms <strong>of</strong><br />
perf<strong>or</strong>mance status, serum PSA level, grade <strong>of</strong><br />
bone pain, type <strong>of</strong> progression, sites <strong>of</strong> secondary<br />
disease, race and age.<br />
F<strong>or</strong> inclusion in <strong>the</strong> trial, patients had to have<br />
progressive metastatic disease, despite androgenablative<br />
<strong>the</strong>rapy and cessation <strong>of</strong> anti-androgen<br />
<strong>treatment</strong>; progression <strong>of</strong> a bi-dimensionally<br />
measurable lesion as assessed <strong>with</strong>in 28 days<br />
bef<strong>or</strong>e study registration, progression <strong>of</strong> disease<br />
that could be evaluated but not measured as<br />
assessed <strong>with</strong>in 42 days bef<strong>or</strong>e registration, <strong>or</strong> an<br />
increase in serum PSA level over <strong>the</strong> baseline level<br />
in at least two consecutive samples obtained at<br />
least 7 days apart.<br />
Patients were also required to have a SWOG<br />
perf<strong>or</strong>mance status sc<strong>or</strong>e <strong>of</strong> 0–2 (3 was allowed if<br />
due to bone pain) and adequate renal, hepatic and<br />
cardiac function.<br />
The median length <strong>of</strong> follow-up was 32 months.<br />
Six patients in <strong>the</strong> docetaxel group and four<br />
patients in <strong>the</strong> mitoxantrone group did not receive<br />
<strong>the</strong> assigned <strong>treatment</strong>. One patient in <strong>the</strong><br />
One-dose Two-dose Mitoxantrone Comparison<br />
docetaxel (A) docetaxel (B) (C)<br />
M<strong>or</strong>tality A vs C: HR = 0.94<br />
(95% CI: 0.29 to 1.02)<br />
B vs C: HR = 0.86<br />
(95% CI: 0.68 to 1.08)<br />
Progression-free survival Not enough data<br />
Response rate 9 3 1 Significant difference – not<br />
enough data<br />
QoL response Not rep<strong>or</strong>ted<br />
Pain response 14/43 (33%) 10/42 (24%) 9/42 (21%) A vs C: RR = 1.52<br />
(95% CI: 0.74 to 3.13)<br />
B vs C: RR = 1.11<br />
(95% CI: 0.50 to 2.45)<br />
PSA decline 29/43 (67%) 26/42 (63%) 7/42 (18%) A vs C: RR = 4.05<br />
(95% CI: 1.99 to 8.21)<br />
B vs C: RR = 3.71<br />
(95% CI: 1.82 to 7.58)<br />
Adverse events:<br />
Discontinued 4 in total<br />
Grade 3/4 25 a 4 a 27 a<br />
Died 1<br />
a May not be mutually exclusive.<br />
mitoxantrone group also received intermittent<br />
radio<strong>the</strong>rapy, which was a maj<strong>or</strong> protocol<br />
deviation. Two patients in <strong>the</strong> docetaxel group and<br />
four patients in <strong>the</strong> mitoxantrone group<br />
discontinued <strong>treatment</strong> <strong>with</strong>in 1 week.<br />
Quality <strong>of</strong> <strong>the</strong> trial comparing docetaxel plus<br />
estramustine <strong>with</strong> mitoxantrone plus <strong>prednisone</strong><br />
This was a randomised open-label comparative<br />
trial. However, <strong>the</strong> methods <strong>of</strong> randomisation and<br />
concealment <strong>of</strong> allocation were not rep<strong>or</strong>ted and<br />
<strong>the</strong>ref<strong>or</strong>e cannot be assessed f<strong>or</strong> adequacy. The<br />
evaluation <strong>of</strong> <strong>the</strong> trial in relation to study quality is<br />
shown in Appendix 7.<br />
Effectiveness <strong>of</strong> docetaxel plus estramustine<br />
versus mitoxantrone plus <strong>prednisone</strong><br />
Overall survival<br />
Overall survival was <strong>the</strong> primary end-point f<strong>or</strong> <strong>the</strong><br />
trial and was defined as <strong>the</strong> time from <strong>the</strong> date <strong>of</strong><br />
randomisation to <strong>the</strong> date <strong>of</strong> death from any cause<br />
<strong>or</strong> cens<strong>or</strong>ed at <strong>the</strong> date <strong>of</strong> last contact. After a<br />
median follow-up <strong>of</strong> 32 months, 217/338 (64%)<br />
patients receiving docetaxel and 235/336 (70%)<br />
patients receiving mitoxantrone had died.<br />
There was a statistically significant benefit in terms<br />
<strong>of</strong> overall survival observed f<strong>or</strong> <strong>the</strong> docetaxel group