Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
142<br />
Appendix 6<br />
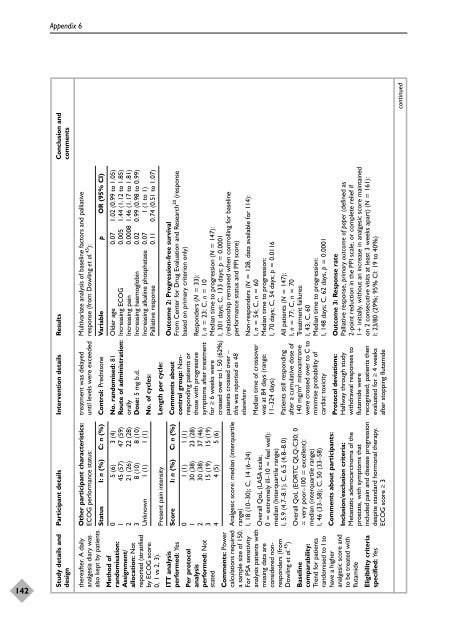
Study details and Participant details Intervention details Results Conclusion and<br />
design comments<br />
Multivariate analysis <strong>of</strong> baseline fact<strong>or</strong>s and palliative<br />
response (from Dowling et al. 45 ):<br />
<strong>treatment</strong> was delayed<br />
until levels were exceeded<br />
O<strong>the</strong>r participant characteristics:<br />
ECOG perf<strong>or</strong>mance status:<br />
<strong>the</strong>reafter. A daily<br />
analgesia diary was<br />
also kept by patients<br />
Variable p OR (95% CI)<br />
Control: Prednisone<br />
Status I: n (%) C: n (%)<br />
Older age 0.07 1.02 (0.99 to 1.05)<br />
Increasing ECOG 0.005 1.44 (1.12 to 1.85)<br />
Increasing pain 0.0008 1.46 (1.17 to 1.81)<br />
Increasing haemoglobin 0.02 0.99 (0.98 to 0.99)<br />
Increasing alkaline phosphatase 0.07 1 (1 to 1)<br />
Palliative response 0.11 0.74 (0.51 to 1.07)<br />
No. randomised: 81<br />
Route <strong>of</strong> administration:<br />
<strong>or</strong>ally<br />
Dose: 5 mg b.d.<br />
0 5 (6) 3 (4)<br />
1 45 (57) 47 (59)<br />
2 21 (26) 22 (28)<br />
3 8 (10) 8 (10)<br />
Unknown 1 (1) 1 (1)<br />
No. <strong>of</strong> cycles:<br />
Method <strong>of</strong><br />
randomisation:<br />
Assignment/<br />
allocation: Not<br />
rep<strong>or</strong>ted (stratified<br />
by ECOG sc<strong>or</strong>e:<br />
0, 1 vs 2, 3).<br />
Length per cycle:<br />
Present pain intensity<br />
Outcome 2: Progression-free survival<br />
From Center f<strong>or</strong> Drug Evaluation and Research20 (response<br />
based on primary criterion only)<br />
Sc<strong>or</strong>e I: n (%) C: n (%)<br />
ITT analysis<br />
perf<strong>or</strong>med: Yes<br />
Responders (N = 33):<br />
I, n = 23; C, n = 10<br />
Median time to progression (N = 147):<br />
I, 301 days; C, 133 days; p = 0.0001<br />
(relationship remained when controlling f<strong>or</strong> baseline<br />
perf<strong>or</strong>mance status and PPI sc<strong>or</strong>e)<br />
Comments about<br />
control group: Nonresponding<br />
patients <strong>or</strong><br />
those <strong>with</strong> progressive<br />
symptoms after <strong>treatment</strong><br />
f<strong>or</strong> ≥ 6 weeks were<br />
crossed over to I. 50 (62%)<br />
patients crossed over –<br />
this was rep<strong>or</strong>ted as 48<br />
elsewhere<br />
0 1 (1) 1 (1)<br />
1 30 (38) 23 (28)<br />
2 30 (38) 37 (46)<br />
3 15 (19) 15 (19)<br />
4 4 (5) 5 (6)<br />
Per protocol<br />
analysis<br />
perf<strong>or</strong>med: Not<br />
stated<br />
Analgesic sc<strong>or</strong>e: median (interquartile<br />
range)<br />
I, 18 (10–30); C, 14 (6–24)<br />
Non-responders (N = 128, data available f<strong>or</strong> 114):<br />
I, n = 54; C, n = 60<br />
Median time to progression:<br />
I, 70 days; C, 54 days; p = 0.0116<br />
Median time <strong>of</strong> crossover<br />
was at 84 days (range:<br />
11–324 days)<br />
Overall QoL (LASA scale;<br />
0 = extremely ill–10 = feel well):<br />
median (Interquartile range)<br />
I, 5.9 (4.7–8.1); C, 6.5 (4.8–8.0)<br />
Comments: Power<br />
calculations required<br />
a sample size <strong>of</strong> 150.<br />
F<strong>or</strong> PSA sensitivity<br />
analysis patients <strong>with</strong><br />
missing data are<br />
considered nonresponders<br />
(from<br />
Dowling et al. 45 )<br />
All patients (N = 147):<br />
I, n = 77; C, n = 70<br />
Treatment failures:<br />
I, 43; C, 60<br />
Median time to progression:<br />
I, 148 days; C, 62 days, p = 0.0001<br />
Outcome 3: Response rate<br />
Palliative response, primary outcome <strong>of</strong> paper (defined as<br />
2-point reduction in <strong>the</strong> PPI scale, <strong>or</strong> complete relief if<br />
1+ initially, <strong>with</strong>out an increase in analgesic sc<strong>or</strong>e maintained<br />
on 2 consecutive visits at least 3 weeks apart) (N = 161):<br />
I: 23/80 (29%; 95% CI: 19 to 40%)<br />
Patients still responding<br />
after a cumulative dose <strong>of</strong><br />
140 mg/m 2 mitoxantrone<br />
were crossed over to C to<br />
minimise probability <strong>of</strong><br />
cardiac toxicity<br />
Overall QoL (EORTC QLQ-C30; 0<br />
= very po<strong>or</strong>–100 = excellent):<br />
median (interquartile range)<br />
I, 46 (33–58); C, 50 (33–58)<br />
Protocol deviations:<br />
Halfway through study<br />
<strong>with</strong>drawal responses to<br />
flutamide were<br />
recognised; patients <strong>the</strong>n<br />
evaluated f<strong>or</strong> ≥ 4 weeks<br />
after stopping flutamide<br />
Comments about participants:<br />
Baseline<br />
comparability:<br />
Trend f<strong>or</strong> patients<br />
randomised to I to<br />
have a higher<br />
analgesic sc<strong>or</strong>e and<br />
to be treated <strong>with</strong><br />
flutamide<br />
Inclusion/exclusion criteria:<br />
Metastatic adenocarcinoma <strong>of</strong> <strong>the</strong><br />
prostate, <strong>with</strong> symptoms that<br />
included pain and disease progression<br />
despite standard h<strong>or</strong>monal <strong>the</strong>rapy.<br />
ECOG sc<strong>or</strong>e ≥ 3<br />
Eligibility criteria<br />
specified: Yes<br />
continued