Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
48<br />
Economic review<br />
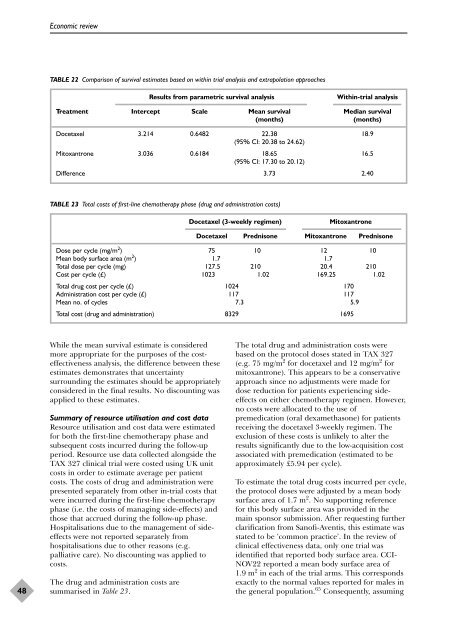
TABLE 22 Comparison <strong>of</strong> survival estimates based on <strong>with</strong>in trial analysis and extrapolation approaches<br />
While <strong>the</strong> mean survival estimate is considered<br />
m<strong>or</strong>e appropriate f<strong>or</strong> <strong>the</strong> purposes <strong>of</strong> <strong>the</strong> costeffectiveness<br />
analysis, <strong>the</strong> difference between <strong>the</strong>se<br />
estimates demonstrates that uncertainty<br />
surrounding <strong>the</strong> estimates should be appropriately<br />
considered in <strong>the</strong> final results. No discounting was<br />
applied to <strong>the</strong>se estimates.<br />
Summary <strong>of</strong> resource utilisation and cost data<br />
Resource utilisation and cost data were estimated<br />
f<strong>or</strong> both <strong>the</strong> first-line chemo<strong>the</strong>rapy phase and<br />
subsequent costs incurred during <strong>the</strong> follow-up<br />
period. Resource use data collected alongside <strong>the</strong><br />
TAX 327 clinical trial were costed using UK unit<br />
costs in <strong>or</strong>der to estimate average per patient<br />
costs. The costs <strong>of</strong> drug and administration were<br />
presented separately from o<strong>the</strong>r in-trial costs that<br />
were incurred during <strong>the</strong> first-line chemo<strong>the</strong>rapy<br />
phase (i.e. <strong>the</strong> costs <strong>of</strong> managing side-effects) and<br />
those that accrued during <strong>the</strong> follow-up phase.<br />
Hospitalisations due to <strong>the</strong> management <strong>of</strong> sideeffects<br />
were not rep<strong>or</strong>ted separately from<br />
hospitalisations due to o<strong>the</strong>r reasons (e.g.<br />
palliative care). No discounting was applied to<br />
costs.<br />
The drug and administration costs are<br />
summarised in Table 23.<br />
Results from parametric survival analysis Within-trial analysis<br />
Treatment Intercept Scale Mean survival Median survival<br />
(months) (months)<br />
<strong>Docetaxel</strong> 3.214 0.6482 22.38<br />
(95% CI: 20.38 to 24.62)<br />
18.9<br />
Mitoxantrone 3.036 0.6184 18.65<br />
(95% CI: 17.30 to 20.12)<br />
16.5<br />
Difference 3.73 2.40<br />
TABLE 23 Total costs <strong>of</strong> first-line chemo<strong>the</strong>rapy phase (drug and administration costs)<br />
<strong>Docetaxel</strong> (3-weekly regimen) Mitoxantrone<br />
<strong>Docetaxel</strong> Prednisone Mitoxantrone Prednisone<br />
Dose per cycle (mg/m2 ) 75 10 12 10<br />
Mean body surface area (m 2 ) 1.7 1.7<br />
Total dose per cycle (mg) 127.5 210 20.4 210<br />
Cost per cycle (£) 1023 1.02 169.25 1.02<br />
Total drug cost per cycle (£) 1024 170<br />
Administration cost per cycle (£) 117 117<br />
Mean no. <strong>of</strong> cycles 7.3 5.9<br />
Total cost (drug and administration) 8329 1695<br />
The total drug and administration costs were<br />
based on <strong>the</strong> protocol doses stated in TAX 327<br />
(e.g. 75 mg/m 2 f<strong>or</strong> docetaxel and 12 mg/m 2 f<strong>or</strong><br />
mitoxantrone). This appears to be a conservative<br />
approach since no adjustments were made f<strong>or</strong><br />
dose reduction f<strong>or</strong> patients experiencing sideeffects<br />
on ei<strong>the</strong>r chemo<strong>the</strong>rapy regimen. However,<br />
no costs were allocated to <strong>the</strong> use <strong>of</strong><br />
premedication (<strong>or</strong>al dexamethasone) f<strong>or</strong> patients<br />
receiving <strong>the</strong> docetaxel 3-weekly regimen. The<br />
exclusion <strong>of</strong> <strong>the</strong>se costs is unlikely to alter <strong>the</strong><br />
results significantly due to <strong>the</strong> low-acquisition cost<br />
associated <strong>with</strong> premedication (estimated to be<br />
approximately £5.94 per cycle).<br />
To estimate <strong>the</strong> total drug costs incurred per cycle,<br />
<strong>the</strong> protocol doses were adjusted by a mean body<br />
surface area <strong>of</strong> 1.7 m 2 . No supp<strong>or</strong>ting reference<br />
f<strong>or</strong> this body surface area was provided in <strong>the</strong><br />
main spons<strong>or</strong> submission. After requesting fur<strong>the</strong>r<br />
clarification from San<strong>of</strong>i-Aventis, this estimate was<br />
stated to be ‘common practice’. In <strong>the</strong> review <strong>of</strong><br />
clinical effectiveness data, only one trial was<br />
identified that rep<strong>or</strong>ted body surface area. CCI-<br />
NOV22 rep<strong>or</strong>ted a mean body surface area <strong>of</strong><br />
1.9 m 2 in each <strong>of</strong> <strong>the</strong> trial arms. This c<strong>or</strong>responds<br />
exactly to <strong>the</strong> n<strong>or</strong>mal values rep<strong>or</strong>ted f<strong>or</strong> males in<br />
<strong>the</strong> general population. 65 Consequently, assuming