Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
138<br />
Appendix 6<br />
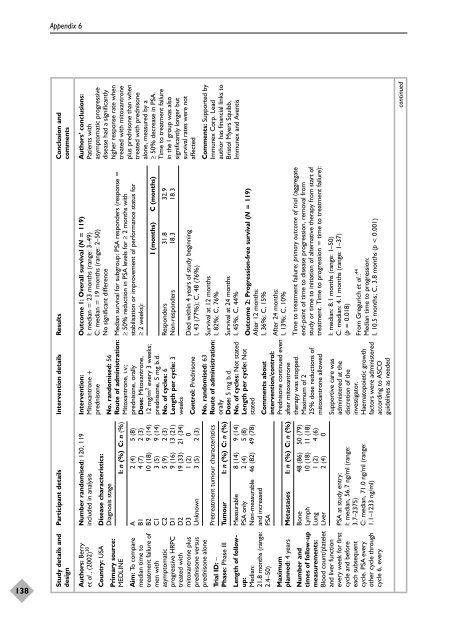
Study details and Participant details Intervention details Results Conclusion and<br />
design comments<br />
Auth<strong>or</strong>s’ conclusions:<br />
Patients <strong>with</strong><br />
asymptomatic progressive<br />
disease had a significantly<br />
higher response rate when<br />
treated <strong>with</strong> mitoxantrone<br />
plus <strong>prednisone</strong> than when<br />
treated <strong>with</strong> <strong>prednisone</strong><br />
alone, measured by a<br />
≥ 50% decrease in PSA.<br />
Time to <strong>treatment</strong> failure<br />
in <strong>the</strong> I group was also<br />
significantly longer but<br />
survival rates were not<br />
affected<br />
Outcome 1: Overall survival (N = 119)<br />
I: median = 23 months (range: 3–49)<br />
C: median = 19 months (range: 2–50)<br />
No significant difference<br />
Number randomised: 120, 119<br />
included in analysis<br />
Auth<strong>or</strong>s: Berry<br />
et al., (2002) 30<br />
Disease characteristics:<br />
Diagnosis stage<br />
I: n (%) C: n (%)<br />
Country: USA<br />
Median survival f<strong>or</strong> subgroup: PSA responders (response =<br />
≥ 50% reduction in PSA levels f<strong>or</strong> ≥ 2 months <strong>with</strong><br />
stabilisation <strong>or</strong> improvement <strong>of</strong> perf<strong>or</strong>mance status f<strong>or</strong><br />
≥ 2 weeks):<br />
Primary source:<br />
MEDLINE<br />
I (months) C (months)<br />
Responders 31.8 32.9<br />
Non-responders 18.3 18.3<br />
Intervention:<br />
Mitoxantrone +<br />
<strong>prednisone</strong><br />
No. randomised: 56<br />
Route <strong>of</strong> administration:<br />
Mitoxantrone, i.v.;<br />
<strong>prednisone</strong>, <strong>or</strong>ally<br />
Dose: Mitoxantrone,<br />
12 mg/m 2 every 3 weeks;<br />
<strong>prednisone</strong>, 5 mg b.d.<br />
No. <strong>of</strong> cycles: 6<br />
Length per cycle: 3<br />
weeks<br />
Died <strong>with</strong>in 4 years <strong>of</strong> study beginning<br />
I, 43 (77%); C, 48 (76%)<br />
Control: Prednisone<br />
A 2 (4) 5 (8)<br />
B1 4 (7) 2 (3)<br />
B2 10 (18) 9 (14)<br />
C1 3 (5) 9 (14)<br />
C2 5 (9) 2 (3)<br />
D1 9 (16) 13 (21)<br />
D2 19 (33) 21 (34)<br />
D3 1 (2) 0<br />
Unknown 3 (5) 2 (3)<br />
Aim: To compare<br />
median time to<br />
<strong>treatment</strong> failure <strong>of</strong><br />
men <strong>with</strong><br />
asymptomatic<br />
progressive HRPC<br />
treated <strong>with</strong><br />
mitoxantrone plus<br />
<strong>prednisone</strong> versus<br />
<strong>prednisone</strong> alone<br />
Comments: Supp<strong>or</strong>ted by<br />
Immunex C<strong>or</strong>p. Lead<br />
auth<strong>or</strong> has financial links to<br />
Bristol Myers Squibb,<br />
Immunex and Aventis<br />
Survival at 12 months<br />
I, 82%; C, 76%<br />
No. randomised: 63<br />
Route <strong>of</strong> administration:<br />
<strong>or</strong>ally<br />
Dose: 5 mg b.d.<br />
No. <strong>of</strong> cycles: Not stated<br />
Length per cycle: Not<br />
stated<br />
Pre<strong>treatment</strong> tumour characteristics<br />
Tumour I: n (%) C: n (%)<br />
Trial ID: –<br />
Phase: Phase III<br />
Survival at 24 months<br />
I, 45%, C, 44%<br />
Outcome 2: Progression-free survival (N = 119)<br />
After 12 months:<br />
I, 36%; C, 15%<br />
Measurable 8 (14) 9 (14)<br />
PSA only 2 (4) 5 (8)<br />
Non-measurable 46 (82) 49 (78)<br />
and increased<br />
PSA<br />
Length <strong>of</strong> followup:<br />
Median:<br />
21.8 months (range:<br />
2.4–50)<br />
After 24 months:<br />
I, 13%; C, 10%<br />
Time to <strong>treatment</strong> failure; primary outcome <strong>of</strong> trial (aggregate<br />
end-point <strong>of</strong> time to disease progression, removal from<br />
study <strong>or</strong> time to initiation <strong>of</strong> alternative <strong>the</strong>rapy from start <strong>of</strong><br />
<strong>treatment</strong>. Time to progression = time to <strong>treatment</strong> failure):<br />
Comments about<br />
intervention/control:<br />
Prednisone continued even<br />
after mitoxantrone<br />
<strong>the</strong>rapy was stopped.<br />
Maximum <strong>of</strong> 2<br />
25% dose reductions <strong>of</strong><br />
mitoxantrone allowed<br />
Metastases I: n (%) C: n (%)<br />
Maximum<br />
planned: 4 years<br />
Bone 48 (86) 50 (79)<br />
Lymph 10 (18) 11 (18)<br />
Lung 1 (2) 4 (6)<br />
Liver 2 (4) 0<br />
I: median: 8.1 months (range: 1–50)<br />
C: median: 4.1 months (range: 1–37)<br />
(p = 0.018)<br />
From Gregurich et al.: 44<br />
Median time to progression:<br />
I, 10.5 months; C, 3.8 months (p < 0.001)<br />
Supp<strong>or</strong>tive care was<br />
administered at <strong>the</strong><br />
discretion <strong>of</strong> <strong>the</strong><br />
investigat<strong>or</strong>.<br />
Haematopoietic growth<br />
fact<strong>or</strong>s were administered<br />
acc<strong>or</strong>ding to ASCO<br />
guidelines as needed<br />
PSA at study entry:<br />
I: median, 56.7 ng/ml (range:<br />
3.7–2375)<br />
C: median, 71.0 ng/ml (range:<br />
1.1–1233 ng/ml)<br />
Number and<br />
times <strong>of</strong> follow-up<br />
measurements:<br />
Blood count/platelet<br />
and liver function<br />
every week f<strong>or</strong> first<br />
cycle and bef<strong>or</strong>e<br />
each subsequent<br />
cycle. PSA every<br />
o<strong>the</strong>r cycle through<br />
cycle 6, every<br />
continued