Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
70<br />
Economic model<br />
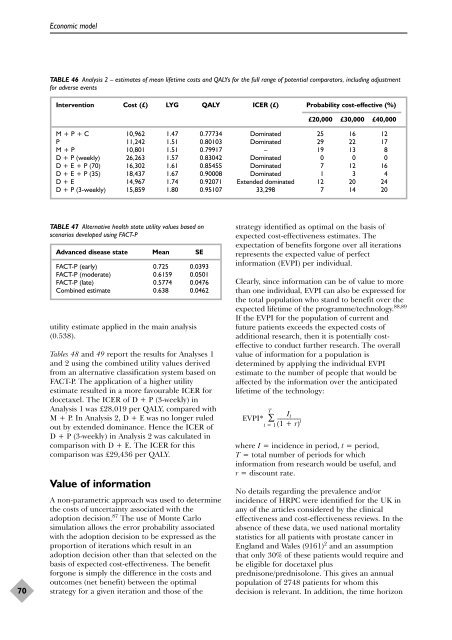
TABLE 46 Analysis 2 – estimates <strong>of</strong> mean lifetime costs and QALYs f<strong>or</strong> <strong>the</strong> full range <strong>of</strong> potential comparat<strong>or</strong>s, including adjustment<br />
f<strong>or</strong> adverse events<br />
Intervention Cost (£) LYG QALY ICER (£) Probability cost-effective (%)<br />
utility estimate applied in <strong>the</strong> main analysis<br />
(0.538).<br />
Tables 48 and 49 rep<strong>or</strong>t <strong>the</strong> results f<strong>or</strong> Analyses 1<br />
and 2 using <strong>the</strong> combined utility values derived<br />
from an alternative classification system based on<br />
FACT-P. The application <strong>of</strong> a higher utility<br />
estimate resulted in a m<strong>or</strong>e favourable ICER f<strong>or</strong><br />
docetaxel. The ICER <strong>of</strong> D + P (3-weekly) in<br />
Analysis 1 was £28,019 per QALY, compared <strong>with</strong><br />
M + P. In Analysis 2, D + E was no longer ruled<br />
out by extended dominance. Hence <strong>the</strong> ICER <strong>of</strong><br />
D + P (3-weekly) in Analysis 2 was calculated in<br />
comparison <strong>with</strong> D + E. The ICER f<strong>or</strong> this<br />
comparison was £29,436 per QALY.<br />
Value <strong>of</strong> inf<strong>or</strong>mation<br />
A non-parametric approach was used to determine<br />
<strong>the</strong> costs <strong>of</strong> uncertainty associated <strong>with</strong> <strong>the</strong><br />
adoption decision. 87 The use <strong>of</strong> Monte Carlo<br />
simulation allows <strong>the</strong> err<strong>or</strong> probability associated<br />
<strong>with</strong> <strong>the</strong> adoption decision to be expressed as <strong>the</strong><br />
prop<strong>or</strong>tion <strong>of</strong> iterations which result in an<br />
adoption decision o<strong>the</strong>r than that selected on <strong>the</strong><br />
basis <strong>of</strong> expected cost-effectiveness. The benefit<br />
f<strong>or</strong>gone is simply <strong>the</strong> difference in <strong>the</strong> costs and<br />
outcomes (net benefit) between <strong>the</strong> optimal<br />
strategy f<strong>or</strong> a given iteration and those <strong>of</strong> <strong>the</strong><br />
strategy identified as optimal on <strong>the</strong> basis <strong>of</strong><br />
expected cost-effectiveness estimates. The<br />
expectation <strong>of</strong> benefits f<strong>or</strong>gone over all iterations<br />
represents <strong>the</strong> expected value <strong>of</strong> perfect<br />
inf<strong>or</strong>mation (EVPI) per individual.<br />
Clearly, since inf<strong>or</strong>mation can be <strong>of</strong> value to m<strong>or</strong>e<br />
than one individual, EVPI can also be expressed f<strong>or</strong><br />
<strong>the</strong> total population who stand to benefit over <strong>the</strong><br />
expected lifetime <strong>of</strong> <strong>the</strong> programme/technology. 88,89<br />
If <strong>the</strong> EVPI f<strong>or</strong> <strong>the</strong> population <strong>of</strong> current and<br />
future patients exceeds <strong>the</strong> expected costs <strong>of</strong><br />
additional research, <strong>the</strong>n it is potentially costeffective<br />
to conduct fur<strong>the</strong>r research. The overall<br />
value <strong>of</strong> inf<strong>or</strong>mation f<strong>or</strong> a population is<br />
determined by applying <strong>the</strong> individual EVPI<br />
estimate to <strong>the</strong> number <strong>of</strong> people that would be<br />
affected by <strong>the</strong> inf<strong>or</strong>mation over <strong>the</strong> anticipated<br />
lifetime <strong>of</strong> <strong>the</strong> technology:<br />
T<br />
It EVPI* ∑ –––––––<br />
t = 1(1 + r) t<br />
£20,000 £30,000 £40,000<br />
M + P + C 10,962 1.47 0.77734 Dominated 25 16 12<br />
P 11,242 1.51 0.80103 Dominated 29 22 17<br />
M + P 10,801 1.51 0.79917 – 19 13 8<br />
D + P (weekly) 26,263 1.57 0.83042 Dominated 0 0 0<br />
D + E + P (70) 16,302 1.61 0.85455 Dominated 7 12 16<br />
D + E + P (35) 18,437 1.67 0.90008 Dominated 1 3 4<br />
D + E 14,967 1.74 0.92071 Extended dominated 12 20 24<br />
D + P (3-weekly) 15,859 1.80 0.95107 33,298 7 14 20<br />
TABLE 47 Alternative health state utility values based on<br />
scenarios developed using FACT-P<br />
Advanced disease state Mean SE<br />
FACT-P (early) 0.725 0.0393<br />
FACT-P (moderate) 0.6159 0.0501<br />
FACT-P (late) 0.5774 0.0476<br />
Combined estimate 0.638 0.0462<br />
where I = incidence in period, t = period,<br />
T = total number <strong>of</strong> periods f<strong>or</strong> which<br />
inf<strong>or</strong>mation from research would be useful, and<br />
r = discount rate.<br />
No details regarding <strong>the</strong> prevalence and/<strong>or</strong><br />
incidence <strong>of</strong> HRPC were identified f<strong>or</strong> <strong>the</strong> UK in<br />
any <strong>of</strong> <strong>the</strong> articles considered by <strong>the</strong> clinical<br />
effectiveness and cost-effectiveness reviews. In <strong>the</strong><br />
absence <strong>of</strong> <strong>the</strong>se data, we used national m<strong>or</strong>tality<br />
statistics f<strong>or</strong> all patients <strong>with</strong> prostate cancer in<br />
England and Wales (9161) 2 and an assumption<br />
that only 30% <strong>of</strong> <strong>the</strong>se patients would require and<br />
be eligible f<strong>or</strong> docetaxel plus<br />
<strong>prednisone</strong>/<strong>prednisolone</strong>. This gives an annual<br />
population <strong>of</strong> 2748 patients f<strong>or</strong> whom this<br />
decision is relevant. In addition, <strong>the</strong> time h<strong>or</strong>izon