Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
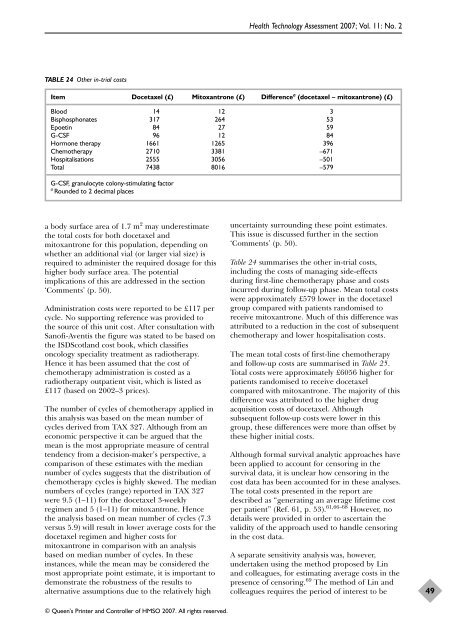
TABLE 24 O<strong>the</strong>r in-trial costs<br />
a body surface area <strong>of</strong> 1.7 m 2 may underestimate<br />
<strong>the</strong> total costs f<strong>or</strong> both docetaxel and<br />
mitoxantrone f<strong>or</strong> this population, depending on<br />
whe<strong>the</strong>r an additional vial (<strong>or</strong> larger vial size) is<br />
required to administer <strong>the</strong> required dosage f<strong>or</strong> this<br />
higher body surface area. The potential<br />
implications <strong>of</strong> this are addressed in <strong>the</strong> section<br />
‘Comments’ (p. 50).<br />
Administration costs were rep<strong>or</strong>ted to be £117 per<br />
cycle. No supp<strong>or</strong>ting reference was provided to<br />
<strong>the</strong> source <strong>of</strong> this unit cost. After consultation <strong>with</strong><br />
San<strong>of</strong>i-Aventis <strong>the</strong> figure was stated to be based on<br />
<strong>the</strong> ISDScotland cost book, which classifies<br />
oncology speciality <strong>treatment</strong> as radio<strong>the</strong>rapy.<br />
Hence it has been assumed that <strong>the</strong> cost <strong>of</strong><br />
chemo<strong>the</strong>rapy administration is costed as a<br />
radio<strong>the</strong>rapy outpatient visit, which is listed as<br />
£117 (based on 2002–3 prices).<br />
The number <strong>of</strong> cycles <strong>of</strong> chemo<strong>the</strong>rapy applied in<br />
this analysis was based on <strong>the</strong> mean number <strong>of</strong><br />
cycles derived from TAX 327. Although from an<br />
economic perspective it can be argued that <strong>the</strong><br />
mean is <strong>the</strong> most appropriate measure <strong>of</strong> central<br />
tendency from a decision-maker’s perspective, a<br />
comparison <strong>of</strong> <strong>the</strong>se estimates <strong>with</strong> <strong>the</strong> median<br />
number <strong>of</strong> cycles suggests that <strong>the</strong> distribution <strong>of</strong><br />
chemo<strong>the</strong>rapy cycles is highly skewed. The median<br />
numbers <strong>of</strong> cycles (range) rep<strong>or</strong>ted in TAX 327<br />
were 9.5 (1–11) f<strong>or</strong> <strong>the</strong> docetaxel 3-weekly<br />
regimen and 5 (1–11) f<strong>or</strong> mitoxantrone. Hence<br />
<strong>the</strong> analysis based on mean number <strong>of</strong> cycles (7.3<br />
versus 5.9) will result in lower average costs f<strong>or</strong> <strong>the</strong><br />
docetaxel regimen and higher costs f<strong>or</strong><br />
mitoxantrone in comparison <strong>with</strong> an analysis<br />
based on median number <strong>of</strong> cycles. In <strong>the</strong>se<br />
instances, while <strong>the</strong> mean may be considered <strong>the</strong><br />
most appropriate point estimate, it is imp<strong>or</strong>tant to<br />
demonstrate <strong>the</strong> robustness <strong>of</strong> <strong>the</strong> results to<br />
alternative assumptions due to <strong>the</strong> relatively high<br />
Health Technology Assessment 2007; Vol. 11: No. 2<br />
Item <strong>Docetaxel</strong> (£) Mitoxantrone (£) Difference a (docetaxel – mitoxantrone) (£)<br />
Blood 14 12 3<br />
Bisphosphonates 317 264 53<br />
Epoetin 84 27 59<br />
G-CSF 96 12 84<br />
H<strong>or</strong>mone <strong>the</strong>rapy 1661 1265 396<br />
Chemo<strong>the</strong>rapy 2710 3381 –671<br />
Hospitalisations 2555 3056 –501<br />
Total 7438 8016 –579<br />
G-CSF, granulocyte colony-stimulating fact<strong>or</strong><br />
a Rounded to 2 decimal places<br />
© Queen’s Printer and Controller <strong>of</strong> HMSO 2007. All rights reserved.<br />
uncertainty surrounding <strong>the</strong>se point estimates.<br />
This issue is discussed fur<strong>the</strong>r in <strong>the</strong> section<br />
‘Comments’ (p. 50).<br />
Table 24 summarises <strong>the</strong> o<strong>the</strong>r in-trial costs,<br />
including <strong>the</strong> costs <strong>of</strong> managing side-effects<br />
during first-line chemo<strong>the</strong>rapy phase and costs<br />
incurred during follow-up phase. Mean total costs<br />
were approximately £579 lower in <strong>the</strong> docetaxel<br />
group compared <strong>with</strong> patients randomised to<br />
receive mitoxantrone. Much <strong>of</strong> this difference was<br />
attributed to a reduction in <strong>the</strong> cost <strong>of</strong> subsequent<br />
chemo<strong>the</strong>rapy and lower hospitalisation costs.<br />
The mean total costs <strong>of</strong> first-line chemo<strong>the</strong>rapy<br />
and follow-up costs are summarised in Table 25.<br />
Total costs were approximately £6056 higher f<strong>or</strong><br />
patients randomised to receive docetaxel<br />
compared <strong>with</strong> mitoxantrone. The maj<strong>or</strong>ity <strong>of</strong> this<br />
difference was attributed to <strong>the</strong> higher drug<br />
acquisition costs <strong>of</strong> docetaxel. Although<br />
subsequent follow-up costs were lower in this<br />
group, <strong>the</strong>se differences were m<strong>or</strong>e than <strong>of</strong>fset by<br />
<strong>the</strong>se higher initial costs.<br />
Although f<strong>or</strong>mal survival analytic approaches have<br />
been applied to account f<strong>or</strong> cens<strong>or</strong>ing in <strong>the</strong><br />
survival data, it is unclear how cens<strong>or</strong>ing in <strong>the</strong><br />
cost data has been accounted f<strong>or</strong> in <strong>the</strong>se analyses.<br />
The total costs presented in <strong>the</strong> rep<strong>or</strong>t are<br />
described as “generating an average lifetime cost<br />
per patient” (Ref. 61, p. 53). 61,66–68 However, no<br />
details were provided in <strong>or</strong>der to ascertain <strong>the</strong><br />
validity <strong>of</strong> <strong>the</strong> approach used to handle cens<strong>or</strong>ing<br />
in <strong>the</strong> cost data.<br />
A separate sensitivity analysis was, however,<br />
undertaken using <strong>the</strong> method proposed by Lin<br />
and colleagues, f<strong>or</strong> estimating average costs in <strong>the</strong><br />
presence <strong>of</strong> cens<strong>or</strong>ing. 69 The method <strong>of</strong> Lin and<br />
colleagues requires <strong>the</strong> period <strong>of</strong> interest to be<br />
49