Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>the</strong>se, 69 patients had measurable tumours. No<br />
complete responses were observed in ei<strong>the</strong>r group.<br />
Partial responses were observed in eight (7%)<br />
patients in <strong>the</strong> mitoxantrone group and five (4%)<br />
patients in <strong>the</strong> hydroc<strong>or</strong>tisone group. There was<br />
no statistically significant difference in terms <strong>of</strong><br />
response rate between groups; RR f<strong>or</strong> response =<br />
1.65 (95% CI: 0.56 to 4.91, p = 0.375).<br />
Health-related quality <strong>of</strong> life HRQoL was assessed<br />
using five instruments. The Functional Living<br />
Index – Cancer (FLIC) <strong>with</strong> sc<strong>or</strong>es ranging from 0<br />
to 7 was used to provide a global assessment <strong>of</strong><br />
QoL. Four o<strong>the</strong>r HRQoL instruments were used to<br />
provide in-depth evaluations <strong>of</strong> cancer-related<br />
symptoms, sexual and urological issues, problems<br />
<strong>with</strong> everyday activities and <strong>the</strong> impact <strong>of</strong> pain on<br />
activities such as sleep and n<strong>or</strong>mal w<strong>or</strong>k.<br />
A total <strong>of</strong> 155 (66%) patients were assessed at<br />
baseline and at least one follow-up. The estimated<br />
<strong>treatment</strong> effects showed that <strong>the</strong>re was no<br />
statistically significant benefit f<strong>or</strong> <strong>the</strong> mitoxantrone<br />
group compared <strong>with</strong> <strong>the</strong> hydroc<strong>or</strong>tisone group in<br />
terms <strong>of</strong> global QoL as assessed by <strong>the</strong> FLIC<br />
questionnaire (p = 0.12). Some <strong>of</strong> <strong>the</strong> items <strong>of</strong> <strong>the</strong><br />
questionnaires did show statistically significant<br />
benefits f<strong>or</strong> <strong>the</strong> mitoxantrone group compared<br />
<strong>with</strong> <strong>the</strong> hydroc<strong>or</strong>tisone group in relation to<br />
emotional state and family disruption (p = 0.04<br />
and 0.02, respectively, as assessed by FLIC) and<br />
severity <strong>of</strong> pain (p = 0.03 as assessed by <strong>the</strong><br />
symptom distress scale).<br />
Pain Data on pain were measured and rep<strong>or</strong>ted<br />
<strong>with</strong> <strong>the</strong> HRQoL assessments. Specific items<br />
assessed by <strong>the</strong> QoL instruments and rep<strong>or</strong>ted<br />
were <strong>the</strong> total impact <strong>of</strong> pain, frequency <strong>of</strong> pain,<br />
severity <strong>of</strong> pain and pain from cancer; only <strong>the</strong><br />
last item showed a tendency f<strong>or</strong> those in <strong>the</strong><br />
hydroc<strong>or</strong>tisone group to have a better QoL than<br />
those in <strong>the</strong> mitoxantrone group.<br />
PSA decline PSA decline was defined as at least a<br />
50% reduction and at least an 80% reduction in<br />
serum PSA from baseline at a follow-up<br />
examination between 4 and 8 weeks. A post hoc<br />
Health Technology Assessment 2007; Vol. 11: No. 2<br />
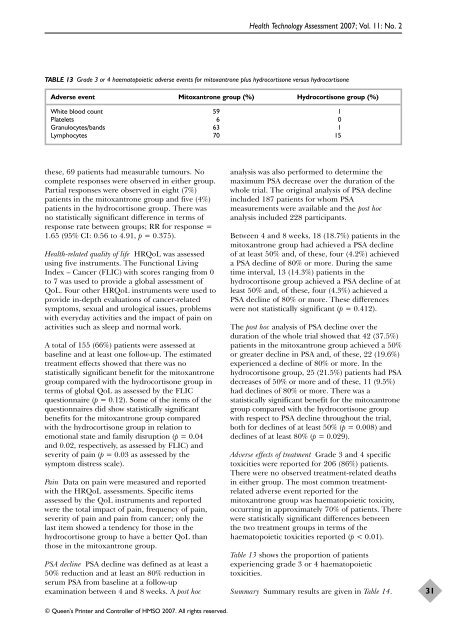
TABLE 13 Grade 3 <strong>or</strong> 4 haematopoietic adverse events f<strong>or</strong> mitoxantrone plus hydroc<strong>or</strong>tisone versus hydroc<strong>or</strong>tisone<br />
Adverse event Mitoxantrone group (%) Hydroc<strong>or</strong>tisone group (%)<br />
White blood count 59 1<br />
Platelets 6 0<br />
Granulocytes/bands 63 1<br />
Lymphocytes 70 15<br />
© Queen’s Printer and Controller <strong>of</strong> HMSO 2007. All rights reserved.<br />
analysis was also perf<strong>or</strong>med to determine <strong>the</strong><br />
maximum PSA decrease over <strong>the</strong> duration <strong>of</strong> <strong>the</strong><br />
whole trial. The <strong>or</strong>iginal analysis <strong>of</strong> PSA decline<br />
included 187 patients f<strong>or</strong> whom PSA<br />
measurements were available and <strong>the</strong> post hoc<br />
analysis included 228 participants.<br />
Between 4 and 8 weeks, 18 (18.7%) patients in <strong>the</strong><br />
mitoxantrone group had achieved a PSA decline<br />
<strong>of</strong> at least 50% and, <strong>of</strong> <strong>the</strong>se, four (4.2%) achieved<br />
a PSA decline <strong>of</strong> 80% <strong>or</strong> m<strong>or</strong>e. During <strong>the</strong> same<br />
time interval, 13 (14.3%) patients in <strong>the</strong><br />
hydroc<strong>or</strong>tisone group achieved a PSA decline <strong>of</strong> at<br />
least 50% and, <strong>of</strong> <strong>the</strong>se, four (4.3%) achieved a<br />
PSA decline <strong>of</strong> 80% <strong>or</strong> m<strong>or</strong>e. These differences<br />
were not statistically significant (p = 0.412).<br />
The post hoc analysis <strong>of</strong> PSA decline over <strong>the</strong><br />
duration <strong>of</strong> <strong>the</strong> whole trial showed that 42 (37.5%)<br />
patients in <strong>the</strong> mitoxantrone group achieved a 50%<br />
<strong>or</strong> greater decline in PSA and, <strong>of</strong> <strong>the</strong>se, 22 (19.6%)<br />
experienced a decline <strong>of</strong> 80% <strong>or</strong> m<strong>or</strong>e. In <strong>the</strong><br />
hydroc<strong>or</strong>tisone group, 25 (21.5%) patients had PSA<br />
decreases <strong>of</strong> 50% <strong>or</strong> m<strong>or</strong>e and <strong>of</strong> <strong>the</strong>se, 11 (9.5%)<br />
had declines <strong>of</strong> 80% <strong>or</strong> m<strong>or</strong>e. There was a<br />
statistically significant benefit f<strong>or</strong> <strong>the</strong> mitoxantrone<br />
group compared <strong>with</strong> <strong>the</strong> hydroc<strong>or</strong>tisone group<br />
<strong>with</strong> respect to PSA decline throughout <strong>the</strong> trial,<br />
both f<strong>or</strong> declines <strong>of</strong> at least 50% (p = 0.008) and<br />
declines <strong>of</strong> at least 80% (p = 0.029).<br />
Adverse effects <strong>of</strong> <strong>treatment</strong> Grade 3 and 4 specific<br />
toxicities were rep<strong>or</strong>ted f<strong>or</strong> 206 (86%) patients.<br />
There were no observed <strong>treatment</strong>-related deaths<br />
in ei<strong>the</strong>r group. The most common <strong>treatment</strong>related<br />
adverse event rep<strong>or</strong>ted f<strong>or</strong> <strong>the</strong><br />
mitoxantrone group was haematopoietic toxicity,<br />
occurring in approximately 70% <strong>of</strong> patients. There<br />
were statistically significant differences between<br />
<strong>the</strong> two <strong>treatment</strong> groups in terms <strong>of</strong> <strong>the</strong><br />
haematopoietic toxicities rep<strong>or</strong>ted (p < 0.01).<br />
Table 13 shows <strong>the</strong> prop<strong>or</strong>tion <strong>of</strong> patients<br />
experiencing grade 3 <strong>or</strong> 4 haematopoietic<br />
toxicities.<br />
Summary Summary results are given in Table 14.<br />
31