Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
170<br />
Appendix 10<br />
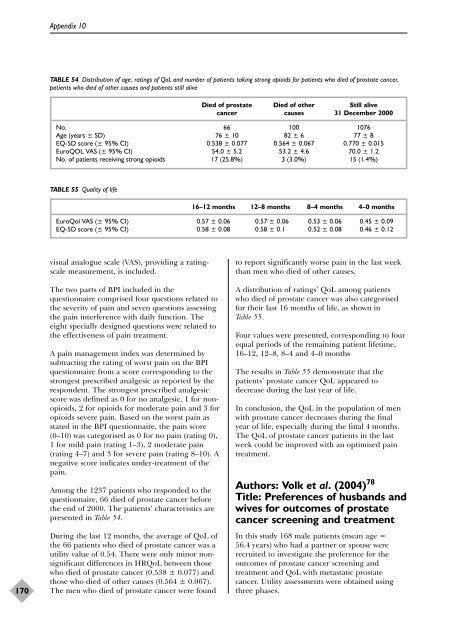
TABLE 54 Distribution <strong>of</strong> age, ratings <strong>of</strong> QoL and number <strong>of</strong> patients taking strong opioids f<strong>or</strong> patients who died <strong>of</strong> prostate cancer,<br />
patients who died <strong>of</strong> o<strong>the</strong>r causes and patients still alive<br />
visual analogue scale (VAS), providing a ratingscale<br />
measurement, is included.<br />
The two parts <strong>of</strong> BPI included in <strong>the</strong><br />
questionnaire comprised four questions related to<br />
<strong>the</strong> severity <strong>of</strong> pain and seven questions assessing<br />
<strong>the</strong> pain interference <strong>with</strong> daily function. The<br />
eight specially designed questions were related to<br />
<strong>the</strong> effectiveness <strong>of</strong> pain <strong>treatment</strong>.<br />
A pain management index was determined by<br />
subtracting <strong>the</strong> rating <strong>of</strong> w<strong>or</strong>st pain on <strong>the</strong> BPI<br />
questionnaire from a sc<strong>or</strong>e c<strong>or</strong>responding to <strong>the</strong><br />
strongest prescribed analgesic as rep<strong>or</strong>ted by <strong>the</strong><br />
respondent. The strongest prescribed analgesic<br />
sc<strong>or</strong>e was defined as 0 f<strong>or</strong> no analgesic, 1 f<strong>or</strong> nonopioids,<br />
2 f<strong>or</strong> opioids f<strong>or</strong> moderate pain and 3 f<strong>or</strong><br />
opioids severe pain. Based on <strong>the</strong> w<strong>or</strong>st pain as<br />
stated in <strong>the</strong> BPI questionnaire, <strong>the</strong> pain sc<strong>or</strong>e<br />
(0–10) was categ<strong>or</strong>ised as 0 f<strong>or</strong> no pain (rating 0),<br />
1 f<strong>or</strong> mild pain (rating 1–3), 2 moderate pain<br />
(rating 4–7) and 3 f<strong>or</strong> severe pain (rating 8–10). A<br />
negative sc<strong>or</strong>e indicates under-<strong>treatment</strong> <strong>of</strong> <strong>the</strong><br />
pain.<br />
Among <strong>the</strong> 1237 patients who responded to <strong>the</strong><br />
questionnaire, 66 died <strong>of</strong> prostate cancer bef<strong>or</strong>e<br />
<strong>the</strong> end <strong>of</strong> 2000. The patients’ characteristics are<br />
presented in Table 54.<br />
During <strong>the</strong> last 12 months, <strong>the</strong> average <strong>of</strong> QoL <strong>of</strong><br />
<strong>the</strong> 66 patients who died <strong>of</strong> prostate cancer was a<br />
utility value <strong>of</strong> 0.54. There were only min<strong>or</strong> nonsignificant<br />
differences in HRQoL between those<br />
who died <strong>of</strong> prostate cancer (0.538 ± 0.077) and<br />
those who died <strong>of</strong> o<strong>the</strong>r causes (0.564 ± 0.067).<br />
The men who died <strong>of</strong> prostate cancer were found<br />
Died <strong>of</strong> prostate Died <strong>of</strong> o<strong>the</strong>r Still alive<br />
cancer causes 31 December 2000<br />
No. 66 100 1076<br />
Age (years ± SD) 76 ± 10 82 ± 6 77 ± 8<br />
EQ-5D sc<strong>or</strong>e (± 95% CI) 0.538 ± 0.077 0.564 ± 0.067 0.770 ± 0.015<br />
EuroQOL VAS (± 95% CI) 54.0 ± 5.2 53.2 ± 4.6 70.0 ± 1.2<br />
No. <strong>of</strong> patients receiving strong opioids 17 (25.8%) 3 (3.0%) 15 (1.4%)<br />
TABLE 55 Quality <strong>of</strong> life<br />
16–12 months 12–8 months 8–4 months 4–0 months<br />
EuroQol VAS (± 95% CI) 0.57 ± 0.06 0.57 ± 0.06 0.53 ± 0.06 0.45 ± 0.09<br />
EQ-5D sc<strong>or</strong>e (± 95% CI) 0.58 ± 0.08 0.58 ± 0.1 0.52 ± 0.08 0.46 ± 0.12<br />
to rep<strong>or</strong>t significantly w<strong>or</strong>se pain in <strong>the</strong> last week<br />
than men who died <strong>of</strong> o<strong>the</strong>r causes.<br />
A distribution <strong>of</strong> ratings’ QoL among patients<br />
who died <strong>of</strong> prostate cancer was also categ<strong>or</strong>ised<br />
f<strong>or</strong> <strong>the</strong>ir last 16 months <strong>of</strong> life, as shown in<br />
Table 55.<br />
Four values were presented, c<strong>or</strong>responding to four<br />
equal periods <strong>of</strong> <strong>the</strong> remaining patient lifetime,<br />
16–12, 12–8, 8–4 and 4–0 months<br />
The results in Table 55 demonstrate that <strong>the</strong><br />
patients’ prostate cancer QoL appeared to<br />
decrease during <strong>the</strong> last year <strong>of</strong> life.<br />
In conclusion, <strong>the</strong> QoL in <strong>the</strong> population <strong>of</strong> men<br />
<strong>with</strong> prostate cancer decreases during <strong>the</strong> final<br />
year <strong>of</strong> life, especially during <strong>the</strong> final 4 months.<br />
The QoL <strong>of</strong> prostate cancer patients in <strong>the</strong> last<br />
week could be improved <strong>with</strong> an optimised pain<br />
<strong>treatment</strong>.<br />
Auth<strong>or</strong>s: Volk et al. (2004) 78<br />
Title: Preferences <strong>of</strong> husbands and<br />
wives f<strong>or</strong> outcomes <strong>of</strong> prostate<br />
cancer screening and <strong>treatment</strong><br />
In this study 168 male patients (mean age =<br />
56.4 years) who had a partner <strong>or</strong> spouse were<br />
recruited to investigate <strong>the</strong> preference f<strong>or</strong> <strong>the</strong><br />
outcomes <strong>of</strong> prostate cancer screening and<br />
<strong>treatment</strong> and QoL <strong>with</strong> metastatic prostate<br />
cancer. Utility assessments were obtained using<br />
three phases.