Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
28<br />
Results<br />
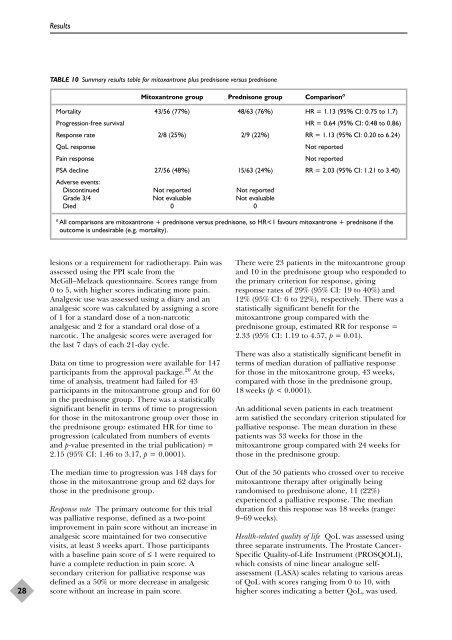
TABLE 10 Summary results table f<strong>or</strong> mitoxantrone plus <strong>prednisone</strong> versus <strong>prednisone</strong><br />
lesions <strong>or</strong> a requirement f<strong>or</strong> radio<strong>the</strong>rapy. Pain was<br />
assessed using <strong>the</strong> PPI scale from <strong>the</strong><br />
McGill–Melzack questionnaire. Sc<strong>or</strong>es range from<br />
0 to 5, <strong>with</strong> higher sc<strong>or</strong>es indicating m<strong>or</strong>e pain.<br />
Analgesic use was assessed using a diary and an<br />
analgesic sc<strong>or</strong>e was calculated by assigning a sc<strong>or</strong>e<br />
<strong>of</strong> 1 f<strong>or</strong> a standard dose <strong>of</strong> a non-narcotic<br />
analgesic and 2 f<strong>or</strong> a standard <strong>or</strong>al dose <strong>of</strong> a<br />
narcotic. The analgesic sc<strong>or</strong>es were averaged f<strong>or</strong><br />
<strong>the</strong> last 7 days <strong>of</strong> each 21-day cycle.<br />
Data on time to progression were available f<strong>or</strong> 147<br />
participants from <strong>the</strong> approval package. 20 At <strong>the</strong><br />
time <strong>of</strong> analysis, <strong>treatment</strong> had failed f<strong>or</strong> 43<br />
participants in <strong>the</strong> mitoxantrone group and f<strong>or</strong> 60<br />
in <strong>the</strong> <strong>prednisone</strong> group. There was a statistically<br />
significant benefit in terms <strong>of</strong> time to progression<br />
f<strong>or</strong> those in <strong>the</strong> mitoxantrone group over those in<br />
<strong>the</strong> <strong>prednisone</strong> group: estimated HR f<strong>or</strong> time to<br />
progression (calculated from numbers <strong>of</strong> events<br />
and p-value presented in <strong>the</strong> trial publication) =<br />
2.15 (95% CI: 1.46 to 3.17, p = 0.0001).<br />
The median time to progression was 148 days f<strong>or</strong><br />
those in <strong>the</strong> mitoxantrone group and 62 days f<strong>or</strong><br />
those in <strong>the</strong> <strong>prednisone</strong> group.<br />
Response rate The primary outcome f<strong>or</strong> this trial<br />
was palliative response, defined as a two-point<br />
improvement in pain sc<strong>or</strong>e <strong>with</strong>out an increase in<br />
analgesic sc<strong>or</strong>e maintained f<strong>or</strong> two consecutive<br />
visits, at least 3 weeks apart. Those participants<br />
<strong>with</strong> a baseline pain sc<strong>or</strong>e <strong>of</strong> ≤ 1 were required to<br />
have a complete reduction in pain sc<strong>or</strong>e. A<br />
secondary criterion f<strong>or</strong> palliative response was<br />
defined as a 50% <strong>or</strong> m<strong>or</strong>e decrease in analgesic<br />
sc<strong>or</strong>e <strong>with</strong>out an increase in pain sc<strong>or</strong>e.<br />
Mitoxantrone group Prednisone group Comparison a<br />
M<strong>or</strong>tality 43/56 (77%) 48/63 (76%) HR = 1.13 (95% CI: 0.75 to 1.7)<br />
Progression-free survival HR = 0.64 (95% CI: 0.48 to 0.86)<br />
Response rate 2/8 (25%) 2/9 (22%) RR = 1.13 (95% CI: 0.20 to 6.24)<br />
QoL response Not rep<strong>or</strong>ted<br />
Pain response Not rep<strong>or</strong>ted<br />
PSA decline<br />
Adverse events:<br />
27/56 (48%) 15/63 (24%) RR = 2.03 (95% CI: 1.21 to 3.40)<br />
Discontinued Not rep<strong>or</strong>ted Not rep<strong>or</strong>ted<br />
Grade 3/4 Not evaluable Not evaluable<br />
Died 0 0<br />
a All comparisons are mitoxantrone + <strong>prednisone</strong> versus <strong>prednisone</strong>, so HR