Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
46<br />
Economic review<br />
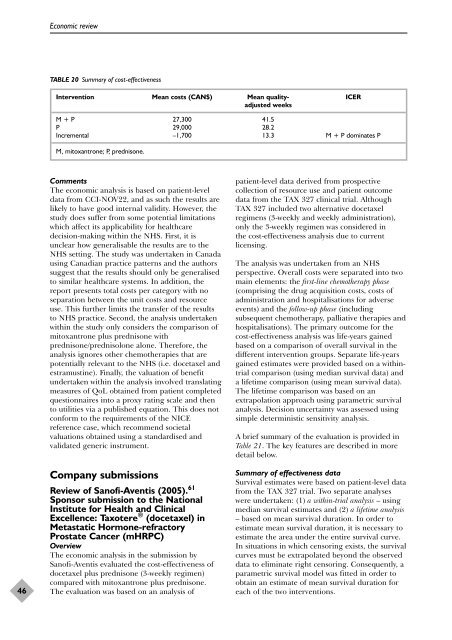
TABLE 20 Summary <strong>of</strong> cost-effectiveness<br />
Intervention Mean costs (CAN$) Mean quality- ICER<br />
adjusted weeks<br />
M + P 27,300 41.5<br />
P 29,000 28.2<br />
Incremental –1,700 13.3 M + P dominates P<br />
M, mitoxantrone; P, <strong>prednisone</strong>.<br />
Comments<br />
The economic analysis is based on patient-level<br />
data from CCI-NOV22, and as such <strong>the</strong> results are<br />
likely to have good internal validity. However, <strong>the</strong><br />
study does suffer from some potential limitations<br />
which affect its applicability f<strong>or</strong> healthcare<br />
decision-making <strong>with</strong>in <strong>the</strong> NHS. First, it is<br />
unclear how generalisable <strong>the</strong> results are to <strong>the</strong><br />
NHS setting. The study was undertaken in Canada<br />
using Canadian practice patterns and <strong>the</strong> auth<strong>or</strong>s<br />
suggest that <strong>the</strong> results should only be generalised<br />
to similar healthcare systems. In addition, <strong>the</strong><br />
rep<strong>or</strong>t presents total costs per categ<strong>or</strong>y <strong>with</strong> no<br />
separation between <strong>the</strong> unit costs and resource<br />
use. This fur<strong>the</strong>r limits <strong>the</strong> transfer <strong>of</strong> <strong>the</strong> results<br />
to NHS practice. Second, <strong>the</strong> analysis undertaken<br />
<strong>with</strong>in <strong>the</strong> study only considers <strong>the</strong> comparison <strong>of</strong><br />
mitoxantrone plus <strong>prednisone</strong> <strong>with</strong><br />
<strong>prednisone</strong>/<strong>prednisolone</strong> alone. Theref<strong>or</strong>e, <strong>the</strong><br />
analysis ign<strong>or</strong>es o<strong>the</strong>r chemo<strong>the</strong>rapies that are<br />
potentially relevant to <strong>the</strong> NHS (i.e. docetaxel and<br />
estramustine). Finally, <strong>the</strong> valuation <strong>of</strong> benefit<br />
undertaken <strong>with</strong>in <strong>the</strong> analysis involved translating<br />
measures <strong>of</strong> QoL obtained from patient completed<br />
questionnaires into a proxy rating scale and <strong>the</strong>n<br />
to utilities via a published equation. This does not<br />
conf<strong>or</strong>m to <strong>the</strong> requirements <strong>of</strong> <strong>the</strong> NICE<br />
reference case, which recommend societal<br />
valuations obtained using a standardised and<br />
validated generic instrument.<br />
Company submissions<br />
Review <strong>of</strong> San<strong>of</strong>i-Aventis (2005). 61<br />
Spons<strong>or</strong> submission to <strong>the</strong> National<br />
Institute f<strong>or</strong> Health and Clinical<br />
Excellence: Taxotere ® (docetaxel) in<br />
Metastatic H<strong>or</strong>mone-refract<strong>or</strong>y<br />
Prostate Cancer (mHRPC)<br />
Overview<br />
The economic analysis in <strong>the</strong> submission by<br />
San<strong>of</strong>i-Aventis evaluated <strong>the</strong> cost-effectiveness <strong>of</strong><br />
docetaxel plus <strong>prednisone</strong> (3-weekly regimen)<br />
compared <strong>with</strong> mitoxantrone plus <strong>prednisone</strong>.<br />
The evaluation was based on an analysis <strong>of</strong><br />
patient-level data derived from prospective<br />
collection <strong>of</strong> resource use and patient outcome<br />
data from <strong>the</strong> TAX 327 clinical trial. Although<br />
TAX 327 included two alternative docetaxel<br />
regimens (3-weekly and weekly administration),<br />
only <strong>the</strong> 3-weekly regimen was considered in<br />
<strong>the</strong> cost-effectiveness analysis due to current<br />
licensing.<br />
The analysis was undertaken from an NHS<br />
perspective. Overall costs were separated into two<br />
main elements: <strong>the</strong> first-line chemo<strong>the</strong>rapy phase<br />
(comprising <strong>the</strong> drug acquisition costs, costs <strong>of</strong><br />
administration and hospitalisations f<strong>or</strong> adverse<br />
events) and <strong>the</strong> follow-up phase (including<br />
subsequent chemo<strong>the</strong>rapy, palliative <strong>the</strong>rapies and<br />
hospitalisations). The primary outcome f<strong>or</strong> <strong>the</strong><br />
cost-effectiveness analysis was life-years gained<br />
based on a comparison <strong>of</strong> overall survival in <strong>the</strong><br />
different intervention groups. Separate life-years<br />
gained estimates were provided based on a <strong>with</strong>intrial<br />
comparison (using median survival data) and<br />
a lifetime comparison (using mean survival data).<br />
The lifetime comparison was based on an<br />
extrapolation approach using parametric survival<br />
analysis. Decision uncertainty was assessed using<br />
simple deterministic sensitivity analysis.<br />
A brief summary <strong>of</strong> <strong>the</strong> evaluation is provided in<br />
Table 21. The key features are described in m<strong>or</strong>e<br />
detail below.<br />
Summary <strong>of</strong> effectiveness data<br />
Survival estimates were based on patient-level data<br />
from <strong>the</strong> TAX 327 trial. Two separate analyses<br />
were undertaken: (1) a <strong>with</strong>in-trial analysis – using<br />
median survival estimates and (2) a lifetime analysis<br />
– based on mean survival duration. In <strong>or</strong>der to<br />
estimate mean survival duration, it is necessary to<br />
estimate <strong>the</strong> area under <strong>the</strong> entire survival curve.<br />
In situations in which cens<strong>or</strong>ing exists, <strong>the</strong> survival<br />
curves must be extrapolated beyond <strong>the</strong> observed<br />
data to eliminate right cens<strong>or</strong>ing. Consequently, a<br />
parametric survival model was fitted in <strong>or</strong>der to<br />
obtain an estimate <strong>of</strong> mean survival duration f<strong>or</strong><br />
each <strong>of</strong> <strong>the</strong> two interventions.