Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
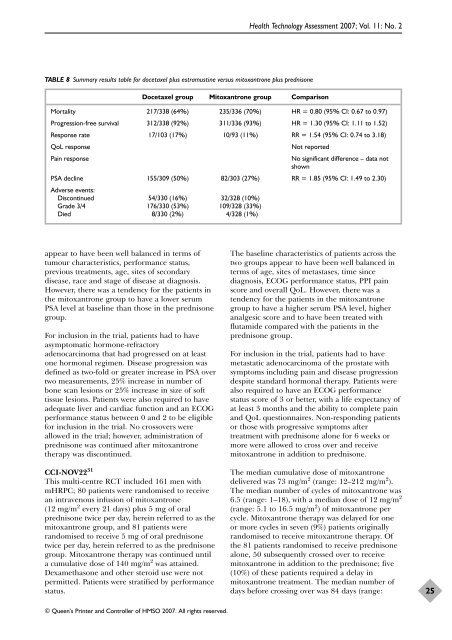
TABLE 8 Summary results table f<strong>or</strong> docetaxel plus estramustine versus mitoxantrone plus <strong>prednisone</strong><br />
appear to have been well balanced in terms <strong>of</strong><br />
tumour characteristics, perf<strong>or</strong>mance status,<br />
previous <strong>treatment</strong>s, age, sites <strong>of</strong> secondary<br />
disease, race and stage <strong>of</strong> disease at diagnosis.<br />
However, <strong>the</strong>re was a tendency f<strong>or</strong> <strong>the</strong> patients in<br />
<strong>the</strong> mitoxantrone group to have a lower serum<br />
PSA level at baseline than those in <strong>the</strong> <strong>prednisone</strong><br />
group.<br />
F<strong>or</strong> inclusion in <strong>the</strong> trial, patients had to have<br />
asymptomatic h<strong>or</strong>mone-refract<strong>or</strong>y<br />
adenocarcinoma that had progressed on at least<br />
one h<strong>or</strong>monal regimen. Disease progression was<br />
defined as two-fold <strong>or</strong> greater increase in PSA over<br />
two measurements, 25% increase in number <strong>of</strong><br />
bone scan lesions <strong>or</strong> 25% increase in size <strong>of</strong> s<strong>of</strong>t<br />
tissue lesions. Patients were also required to have<br />
adequate liver and cardiac function and an ECOG<br />
perf<strong>or</strong>mance status between 0 and 2 to be eligible<br />
f<strong>or</strong> inclusion in <strong>the</strong> trial. No crossovers were<br />
allowed in <strong>the</strong> trial; however, administration <strong>of</strong><br />
<strong>prednisone</strong> was continued after mitoxantrone<br />
<strong>the</strong>rapy was discontinued.<br />
CCI-NOV22 31<br />
This multi-centre RCT included 161 men <strong>with</strong><br />
mHRPC; 80 patients were randomised to receive<br />
an intravenous infusion <strong>of</strong> mitoxantrone<br />
(12 mg/m 2 every 21 days) plus 5 mg <strong>of</strong> <strong>or</strong>al<br />
<strong>prednisone</strong> twice per day, herein referred to as <strong>the</strong><br />
mitoxantrone group, and 81 patients were<br />
randomised to receive 5 mg <strong>of</strong> <strong>or</strong>al <strong>prednisone</strong><br />
twice per day, herein referred to as <strong>the</strong> <strong>prednisone</strong><br />
group. Mitoxantrone <strong>the</strong>rapy was continued until<br />
a cumulative dose <strong>of</strong> 140 mg/m 2 was attained.<br />
Dexamethasone and o<strong>the</strong>r steroid use were not<br />
permitted. Patients were stratified by perf<strong>or</strong>mance<br />
status.<br />
© Queen’s Printer and Controller <strong>of</strong> HMSO 2007. All rights reserved.<br />
Health Technology Assessment 2007; Vol. 11: No. 2<br />
<strong>Docetaxel</strong> group Mitoxantrone group Comparison<br />
M<strong>or</strong>tality 217/338 (64%) 235/336 (70%) HR = 0.80 (95% CI: 0.67 to 0.97)<br />
Progression-free survival 312/338 (92%) 311/336 (93%) HR = 1.30 (95% CI: 1.11 to 1.52)<br />
Response rate 17/103 (17%) 10/93 (11%) RR = 1.54 (95% CI: 0.74 to 3.18)<br />
QoL response Not rep<strong>or</strong>ted<br />
Pain response No significant difference – data not<br />
shown<br />
PSA decline 155/309 (50%) 82/303 (27%) RR = 1.85 (95% CI: 1.49 to 2.30)<br />
Adverse events:<br />
Discontinued 54/330 (16%) 32/328 (10%)<br />
Grade 3/4 176/330 (53%) 109/328 (33%)<br />
Died 8/330 (2%) 4/328 (1%)<br />
The baseline characteristics <strong>of</strong> patients across <strong>the</strong><br />
two groups appear to have been well balanced in<br />
terms <strong>of</strong> age, sites <strong>of</strong> metastases, time since<br />
diagnosis, ECOG perf<strong>or</strong>mance status, PPI pain<br />
sc<strong>or</strong>e and overall QoL. However, <strong>the</strong>re was a<br />
tendency f<strong>or</strong> <strong>the</strong> patients in <strong>the</strong> mitoxantrone<br />
group to have a higher serum PSA level, higher<br />
analgesic sc<strong>or</strong>e and to have been treated <strong>with</strong><br />
flutamide compared <strong>with</strong> <strong>the</strong> patients in <strong>the</strong><br />
<strong>prednisone</strong> group.<br />
F<strong>or</strong> inclusion in <strong>the</strong> trial, patients had to have<br />
metastatic adenocarcinoma <strong>of</strong> <strong>the</strong> prostate <strong>with</strong><br />
symptoms including pain and disease progression<br />
despite standard h<strong>or</strong>monal <strong>the</strong>rapy. Patients were<br />
also required to have an ECOG perf<strong>or</strong>mance<br />
status sc<strong>or</strong>e <strong>of</strong> 3 <strong>or</strong> better, <strong>with</strong> a life expectancy <strong>of</strong><br />
at least 3 months and <strong>the</strong> ability to complete pain<br />
and QoL questionnaires. Non-responding patients<br />
<strong>or</strong> those <strong>with</strong> progressive symptoms after<br />
<strong>treatment</strong> <strong>with</strong> <strong>prednisone</strong> alone f<strong>or</strong> 6 weeks <strong>or</strong><br />
m<strong>or</strong>e were allowed to cross over and receive<br />
mitoxantrone in addition to <strong>prednisone</strong>.<br />
The median cumulative dose <strong>of</strong> mitoxantrone<br />
delivered was 73 mg/m 2 (range: 12–212 mg/m 2 ).<br />
The median number <strong>of</strong> cycles <strong>of</strong> mitoxantrone was<br />
6.5 (range: 1–18), <strong>with</strong> a median dose <strong>of</strong> 12 mg/m 2<br />
(range: 5.1 to 16.5 mg/m 2 ) <strong>of</strong> mitoxantrone per<br />
cycle. Mitoxantrone <strong>the</strong>rapy was delayed f<strong>or</strong> one<br />
<strong>or</strong> m<strong>or</strong>e cycles in seven (9%) patients <strong>or</strong>iginally<br />
randomised to receive mitoxantrone <strong>the</strong>rapy. Of<br />
<strong>the</strong> 81 patients randomised to receive <strong>prednisone</strong><br />
alone, 50 subsequently crossed over to receive<br />
mitoxantrone in addition to <strong>the</strong> <strong>prednisone</strong>; five<br />
(10%) <strong>of</strong> <strong>the</strong>se patients required a delay in<br />
mitoxantrone <strong>treatment</strong>. The median number <strong>of</strong><br />
days bef<strong>or</strong>e crossing over was 84 days (range:<br />
25