Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
126<br />
Appendix 6<br />
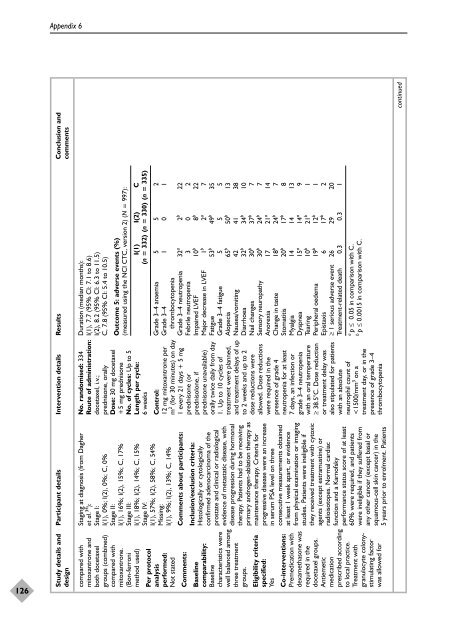
Study details and Participant details Intervention details Results Conclusion and<br />
design comments<br />
Duration (median months):<br />
I(1), 7.7 (95% CI: 7.1 to 8.6)<br />
I(2), 8.2 (95% CI: 6.3 to 11.5)<br />
C, 7.8 (95% CI: 5.4 to 10.5)<br />
Outcome 5: adverse events (%)<br />
(measured using <strong>the</strong> NCI CTC, version 2) (N = 997):<br />
I(1) I(2) C<br />
(n = 332) (n = 330) (n = 335)<br />
No. randomised: 334<br />
Route <strong>of</strong> administration:<br />
docetaxel, i.v.;<br />
<strong>prednisone</strong>, <strong>or</strong>ally<br />
Dose: 30 mg docetaxel<br />
+5 mg <strong>prednisone</strong><br />
No. <strong>of</strong> cycles: Up to 5<br />
Length per cycle:<br />
6 weeks<br />
Staging at diagnosis (from Dagher<br />
et al. 34 ):<br />
Stage I:<br />
I(1), 0%; I(2), 0%; C, 0%<br />
Stage II:<br />
I(1), 16%; I(2), 15%; C, 17%<br />
Stage III:<br />
I(1), 18%; I(2), 14%; C, 15%<br />
Stage IV:<br />
I(1), 57%; I(2), 58%; C, 54%<br />
Missing:<br />
I(1), 9%; I(2), 13%; C, 14%<br />
compared <strong>with</strong><br />
mitoxantrone and<br />
both docetaxel<br />
groups (combined)<br />
compared <strong>with</strong><br />
mitoxantrone.<br />
(Bon-ferroni<br />
method used)<br />
Grade 3–4 anaemia 5 5 2<br />
Grade 3–4 1 0 1<br />
Control:<br />
12 mg mitoxantrone per<br />
m 2 (f<strong>or</strong> 30 minutes) on day<br />
1 every 21 days + 5 mg<br />
<strong>prednisone</strong> (<strong>or</strong><br />
<strong>prednisolone</strong>, if<br />
<strong>prednisone</strong> unavailable)<br />
<strong>or</strong>ally twice daily from day<br />
1. Up to 10 cycles <strong>of</strong><br />
<strong>treatment</strong> were planned,<br />
and <strong>treatment</strong> delays <strong>of</strong> up<br />
to 2 weeks and up to 2<br />
dose reductions were<br />
allowed. Dose reductions<br />
were required in <strong>the</strong><br />
presence <strong>of</strong> grade 4<br />
neutropenia f<strong>or</strong> at least<br />
7 days, an infection <strong>or</strong><br />
grade 3–4 neutropenia<br />
<strong>with</strong> an <strong>or</strong>al temperature<br />
≥ 38.5°C. Dose reduction<br />
<strong>or</strong> <strong>treatment</strong> delay was<br />
also stipulated f<strong>or</strong> patients<br />
<strong>with</strong> an absolute<br />
neutrophil count <strong>of</strong><br />